Abstract
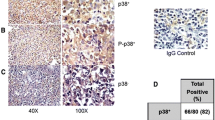
The aims of this study were to investigate FOXP1 expression in nodal and extranodal diffuse large B-cell lymphoma (DLBCL) and its association with the subclassification and other clinicopathologic parameters of DLBCL. Expression of FOXP1, CD10, Bcl6, MUM1, and Bcl2 was detected by immunohistochemistry on tissue microarray sections. The Kaplan–Meier method was used to estimate the overall survival of patients, and the log-rank test was used to compare survival differences between groups with different FOXP1 protein expressions. Expression of FOXP1 was detected in 67.4% (95/141) of DLBCLs. FOXP1 expression in non-GCB (67/90, 74.4%) was significantly higher than that in GCB (28/51, 54.9%) (p < 0.05). FOXP1 expression in MUM1-positive cases (62/81, 76.5%) was significantly higher than that in MUM1-negative cases (33/60, 55%) (p < 0.01). FOXP1 expression was positively correlated with Bcl2 (p < 0.05) in non-GCB among nodal DLBCL cases. Among the extranodal group, patients with FOXP1 expression had a significantly inferior OS compared to those with negative FOXP1 expression (p < 0.05), which was not seen in nodal group. In conclusion, FOXP1 expression might be involved in the tumorigenesis of both nodal and extranodal DLBCL. The most striking finding of this study was that FOXP1 expression had an adverse effect on survival of patients with extranodal DLBCL, which indicated that FOXP1 function might be mediated by different mechanisms in nodal and extranodal DLBCLs. FOXP1 might play a role in the pathogenesis of nodal non-GCB DLBCL through the pathways in which Bcl2 was involved, and it might be a second important biomarker for non-GCB.


Similar content being viewed by others
References
Coiffier B (2001) Diffuse large cell lymphoma. Curr Opin Oncol 13:325–334
Fisher RI, Gaynor ER, Dahlberg S et al (1993) Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 328:1002–1006
Sweetenham JW (2005) Diffuse large B-cell lymphoma: risk stratification and management of relapsed disease. Hematology Am Soc Hematol Educ Program 252–259
Alizadeh AA, Eisen MB, Davis RE et al (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511
Rosenwald A, Wright G, Chan WC et al (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346:1937–1947
Lossos IS, Czerwinski DK, Alizadeh AA et al (2004) Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med 350:1828–1837
Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM (2003) A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci USA 100:9991–9996
Barrans SL, Carter I, Owen RG et al (2002) Germinal center phenotype and bcl-2 expression combined with the International Prognostic Index improves patient risk stratification in diffuse large B-cell lymphoma. Blood 99:1136–1143
Berglund M, Thunberg U, Amini RM et al (2005) Evaluation of immunophenotype in diffuse large B-cell lymphoma and its impact on prognosis. Mod Pathol 18:1113–1120
Colomo L, Lopez-Guillermo A, Perales M et al (2003) Clinical impact of the differentiation profile assessed by immunophenotyping in patients with diffuse large B-cell lymphoma. Blood 101:78–84
Hans CP, Weisenburger DD, Greiner TC et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282
Linderoth J, Jerkeman M, Cavallin-Stahl E, Kvaloy S, Torlakovic E (2003) Immunohistochemical expression of CD23 and CD40 may identify prognostically favorable subgroups of diffuse large B-cell lymphoma: a Nordic Lymphoma Group Study. Clin Cancer Res 9:722–728
Kaufmann E, Knochel W (1996) Five years on the wings of fork head. Mech Dev 57:3–20
Shu W, Yang H, Zhang L, Lu MM, Morrisey EE (2001) Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem 276:27488–27497
Banham AH, Beasley N, Campo E et al (2001) The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res 61:8820–8829
Shaffer AL, Rosenwald A, Staudt LM (2002) Lymphoid malignancies: the dark side of B-cell differentiation. Nat Rev Immunol 2:920–932
Banham AH, Connors JM, Brown PJ et al (2005) Expression of the FOXP1 transcription factor is strongly associated with inferior survival in patients with diffuse large B-cell lymphoma. Clin Cancer Res 11:1065–1072
Barrans SL, Fenton JA, Banham A, Owen RG, Jack AS (2004) Strong expression of FOXP1 identifies a distinct subset of diffuse large B-cell lymphoma (DLBCL) patients with poor outcome. Blood 104:2933–2935
Sagaert X, Paepe PD, Libbrecht L et al (2006) Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol 24:2490–2497
Wlodarska I, Veyt E, De Paepe P et al (2005) FOXP1, a gene highly expressed in a subset of diffuse large B-cell lymphoma, is recurrently targeted by genomic aberrations. Leukemia 19:1299–1305
Wang B, Lin D, Li C, Tucker P (2003) Multiple domains define the expression and regulatory properties of Foxp1 forkhead transcriptional repressors. J Biol Chem 278:24259–24268
Brown P, Marafioti T, Kusec R, Banham A (2005) The FOXP1 transcription factor is expressed in the majority of follicular lymphomas but is rarely expressed in classical and lymphocyte predominant Hodgkin’s lymphoma. J Mol Histol 36:249–256
Iqbal J, Neppalli VT, Wright G et al (2006) BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol 24:961–968
Falini B, Fizzotti M, Pucciarini A et al (2000) A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood 95:2084–2092
Gaidano G, Carbone A (2000) MUM1: a step ahead toward the understanding of lymphoma histogenesis. Leukemia 14:563–566
Mounier N, Briere J, Gisselbrecht C et al (2003) Rituximab plus CHOP (R-CHOP) overcomes bcl-2-associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL). Blood 101:4279–4284
Kusumoto S, Kobayashi Y, Sekiguchi N et al (2005) Diffuse large b-cell lymphoma with extra Bcl-2 gene signals detected by FISH analysis is associated with a “Non-Germinal Center Phenotype”. Am J Surg Pathol 29:1067–1073
Catz SD, Johnson JL (2001) Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene 20:7342–7351
Davis RE, Brown KD, Siebenlist U, Staudt LM (2001) Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 194:1861–1874
Hoefnagel JJ, Mulder MM, Dreef E et al (2006) Expression of B-cell transcription factors in primary cutaneous B-cell lymphoma. Mod Pathol 19:1270–1276
Kodama K, Massone C, Chott A, Metze D, Kerl H, Cerroni L (2005) Primary cutaneous large B-cell lymphomas: clinicopathologic features, classification, and prognostic factors in a large series of patients. Blood 106:2491–2497
Funding
This work was supported by grants from the National Nature Science Funding of China (NSFC) (Code No. 30870985 and Code No. 30973391).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, B., Zhou, X., Li, B. et al. FOXP1 expression and its clinicopathologic significance in nodal and extranodal diffuse large B-cell lymphoma. Ann Hematol 90, 701–708 (2011). https://doi.org/10.1007/s00277-010-1124-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-010-1124-9