Abstract
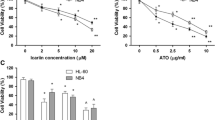
During the last years remission rates of more than 72% for arsenic(III)-oxide (As2O3) treatment in relapsed or refractory acute promyelocytic leukemia have been published. As2O3 is under clinical investigation for therapy of leukemia and solid tumors. Due to the chemical affinity of arsenic and antimony, we analyzed the potency of antimony(III)-oxide (Sb2O3) to exert As2O3-like effects. Based on the same molar concentrations, lower efficacy in apoptosis induction and caspase-independent decrease of mitochondrial membrane potential was observed for Sb2O3. No difference in sensitivity to As2O3 or Sb2O3 was detected in CEM cells when compared to their multiple drug resistant derivatives. Apoptosis was induced by combining sub-apoptotic concentrations of Sb2O3 or As2O3 with sub-apoptotic concentrations of dl-buthionine-[S,R]-sulfoximine (BSO). Other modulators of the cellular redox system showed this effect to a lower extent and enhancement was not consistent for the different cell lines tested. Caspase inhibitors protected cell lines from Sb2O3- and As2O3-induced apoptosis. When BSO was added, the inhibitors lost their protective ability. The ability of modulators of the cellular redox system in clinically applicable concentrations to enhance the apoptotic effects of the two oxides in a synergistic way may be helpful to reduce their toxicity by optimizing their dose.








Similar content being viewed by others
References
Rojewski MT, Korper S, Schrezenmeier H (2004) Arsenic trioxide therapy in acute promyelocytic leukemia and beyond: from bench to bedside. Leuk Lymphoma 45:2387–2401. doi:10.1080/10428190412331272686
Amadori S, Fenaux P, Ludwig H, O’Dwyer M, Sanz M (2005) Use of arsenic trioxide in haematological malignancies: insight into the clinical development of a novel agent. Curr Med Res Opin 21:403–411. doi:10.1185/030079904X20349
Bonati A, Rizzoli V, Lunghi P (2006) Arsenic trioxide in hematological malignancies: the new discovery of an ancient drug. Curr Pharm Biotechnol 7:397–405. doi:10.2174/138920106779116829
Douer D, Tallman MS (2005) Arsenic trioxide: new clinical experience with an old medication in hematologic malignancies. J Clin Oncol 23:2396–2410. doi:10.1200/JCO.2005.10.217
Kalmadi SR, Hussein MA (2006) The emerging role of arsenic trioxide as an immunomodulatory agent in the management of multiple myeloma. Acta Haematol 116:1–7. doi:10.1159/000092341
Muller S, Matunis MJ, Dejean A (1998) Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J 17:61–70. doi:10.1093/emboj/17.1.61
Muller S, Miller WH Jr, Dejean A (1998) Trivalent antimonials induce degradation of the PML-RAR oncoprotein and reorganization of the promyelocytic leukemia nuclear bodies in acute promyelocytic leukemia NB4 cells. Blood 92:4308–4316
Sternsdorf T, Puccetti E, Jensen K et al (1999) PIC-1/SUMO-1-modified PML-retinoic acid receptor alpha mediates arsenic trioxide-induced apoptosis in acute promyelocytic leukemia. Mol Cell Biol 19:5170–5178
Lallemand-Breitenbach V, Zhu J, Puvion F et al (2001) Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11 S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J Exp Med 193:1361–1371. doi:10.1084/jem.193.12.1361
Chen GQ, Shi XG, Tang W et al (1997) Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood 89:3345–3353
Bruserud O, Gjertsen BT, Huang T (2000) Induction of differentiation and apoptosis—a possible strategy in the treatment of adult acute myelogenous leukemia. Oncologist 5:454–462. doi:10.1634/theoncologist.5-6-454
Calleja EM, Warrell RP (2000) Differentiating agents in pediatric malignancies: all-trans-retinoic acid and arsenic in acute promyelocytic leukemia. Curr Oncol Rep 2:519–523. doi:10.1007/s11912-000-0105-x
Huff J, Waalkes M, Nyska A, Chan P (1999) Re: apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst 91:1690–1691. doi:10.1093/jnci/91.19.1690 letter
Zhu XH, Shen YL, Jing YK et al (1999) Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst 91:772–778. doi:10.1093/jnci/91.9.772
Rojewski MT, Körper S, Thiel E, Schrezenmeier H (2004) Depolarization of mitochondria and activation of caspases are common features of arsenic(III)-induced apoptosis in myelogenic and lymphatic cell lines. Chem Res Toxicol 17:119–128. doi:10.1021/tx034104+
Nolte F, Friedrich O, Rojewski M, Fink RH, Schrezenmeier H, Korper S (2004) Depolarisation of the plasma membrane in the arsenic trioxide (As2O3)-and anti-CD95-induced apoptosis in myeloid cells. FEBS Lett 578:85–89. doi:10.1016/j.febslet.2004.10.075
Korper S, Nolte F, Thiel E, Schrezenmeier H, Rojewski MT (2004) The role of mitochondrial targeting in arsenic trioxide-induced apoptosis in myeloid cell lines. Br J Haematol 124:186–189. doi:10.1046/j.1365-2141.2003.04742.x
Tsai S, Hsieh M, Chen L, Liang Y, Lin J, Lin S (2001) Suppression of Fas ligand expression on endothelial cells by arsenite through reactive oxygen species. Toxicol Lett 123:11–19. doi:10.1016/S0378-4274(01) 00373-3
Yi J, Gao F, Shi G, Li H, Shi X, Tang X (2002) Apoptosis susceptibility of tumor cells to arsenic trioxide and the inherent cellular level of reactive oxygen species. Chin Med J (Engl) 115:603–606
Nakagawa Y, Akao Y, Morikawa H et al (2002) Arsenic trioxide-induced apoptosis through oxidative stress in cells of colon cancer cell lines. Life Sci 70:2253–2269. doi:10.1016/S0024-3205(01) 01545-4
Choi YJ, Park JW, Suh SI et al (2002) Arsenic trioxide-induced apoptosis in U937 cells involve generation of reactive oxygen species and inhibition of Akt. Int J Oncol 21:603–610
Rojewski MT, Baldus C, Knauf W, Thiel E, Schrezenmeier H (2002) Dual effects of arsenic trioxide (As2O3) on non-acute promyelocytic leukaemia myeloid cell lines: induction of apoptosis and inhibition of proliferation. Br J Haematol 116:555–563. doi:10.1046/j.0007-1048.2001.03298.x
Martensson J, Meister A (1991) Glutathione deficiency decreases tissue ascorbate levels in newborn rats: ascorbate spares glutathione and protects. Proc Natl Acad Sci USA 88:4656–4660. doi:10.1073/pnas.88.11.4656
Wu D, Cederbaum A (2004) Glutathione depletion in CYP2E1-expressing liver cells induces toxicity due to the activation of p38 mitogen-activated protein kinase and reduction of nuclear factor-kappaB DNA binding activity. Mol Pharmacol 66:749–760. doi:10.1124/mol.104.002048
Macho A, Decaudin D, Castedo M et al (1996) Chloromethyl-X-Rosamine is an aldehyde-fixable potential-sensitive fluorochrome for the detection of early apoptosis. Cytometry 25:333–340. doi:10.1002/(SICI) 1097-0320(19961201) 25:4<333::AID-CYTO4>3.0.CO;2-E
Gilmore K, Wilson M (1999) The use of chloromethyl-X-rosamine (Mitotracker red) to measure loss of mitochondrial membrane potential in apoptotic cells is incompatible with cell fixation. Cytometry 36:355–358. doi:10.1002/(SICI) 1097-0320(19990801) 36:4<355::AID-CYTO11>3.0.CO;2-9
Leira F, Vieites JM, Vieytes MR, Botana LM (2001) Apoptotic events induced by the phosphatase inhibitor okadaic acid in normal human lung fibroblasts. Toxicol In Vitro 15:199–208. doi:10.1016/S0887-2333(01) 00013-3
Buckman JF, Hernandez H, Kress GJ, Votyakova TV, Pal S, Reynolds IJ (2001) MitoTracker labeling in primary neuronal and astrocytic cultures: influence of mitochondrial membrane potential and oxidants. J Neurosci Methods 104:165–176. doi:10.1016/S0165-0270(00) 00340-X
Soignet SL, Maslak P, Wang ZG et al (1998) Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 339:1341–1348. doi:10.1056/NEJM199811053391901
Shen ZX, Chen GQ, Ni JH et al (1997) Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood 89:3354–3360
Kapoor R, Slade DL, Fujimori A, Pommier Y, Harker WG (1995) Altered topoisomerase I expression in two subclones of human CEM leukemia selected for resistance to camptothecin. Oncol Res 7:83–95
Fujimori A, Harker WG, Kohlhagen G, Hoki Y, Pommier Y (1995) Mutation at the catalytic site of topoisomerase I in CEM/C2, a human leukemia cell line resistant to camptothecin. Cancer Res 55:1339–1346
Zhang P, Wang SY, Hu XH (1996) Arsenic trioxide treated 72 cases of acute promyelocytic leukemia. Chin J Hematol 17:58–62
Mathews V, Balasubramanian P, Shaji RV, George B, Chandy M, Srivastava A (2002) Arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: a single center experience. Am J Hematol 70:292–299. doi:10.1002/ajh.10138
Niu C, Yan H, Yu T et al (1999) Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood 94:3315–3324
Spencer A, Firkin F (1999) Arsenic trioxide treatment of relapsed acute promyelocytic leukaemia: initial Australian experience. Aust N Z J Med 29:385–386 letter
Warrell RP Jr, Soignet SL, Maslak P et al (1998) Initial Western study of arsenic trioxide in acute promyelocytic leukemia. J Clin Oncol 17(Suppl.):19
Ohnishi K, Yoshida H, Shigeno K et al (2002) Arsenic trioxide therapy for relapsed or refractory Japanese patients with acute promyelocytic leukemia: need for careful electrocardiogram monitoring. Leukemia 16:617–622. doi:10.1038/sj.leu.2402426
Hu J, Shen ZX, Sun GL, Chen SJ, Wang ZY, Chen Z (1999) Long-term survival and prognostic study in acute promyelocytic leukemia treated with all-trans-retinoic acid, chemotherapy, and As2O3: an experience of 120 patients at a single institution. Int J Hematol 70:248–260
Hu J, Shen Z, Sun H et al (2000) Long-term survey of outcome in acute promyelocytic leukemia. Chin Med J (Engl) 113:107–110. doi:10.3901/JME.2000.08.107
Soignet SL, Frankel SR, Douer D et al (2001) United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol 19:3852–3860
Ni J, Chen G, Shen Z et al (1998) Pharmacokinetics of intravenous arsenic trioxide in the treatment of acute promyelocytic leukemia. Chin Med J (Engl) 111:1107–1110
Soignet SL (2001) Clinical experience of arsenic trioxide in relapsed acute promyelocytic leukemia. Oncologist 6:11–16. doi:10.1634/theoncologist.6-suppl_2-11
Jiong H, Zhixiang S, Wen W, Xiusong L, Guanlin S, Zhenyi W (2000) Long-term survey of outcome in acute promyelocytic leukemia. Chin Med J (Engl) 113:107–110
Camacho LH, Soignet SL, Chanel S et al (2000) Leukocytosis and the retinoic acid syndrome in patients with acute promyelocytic leukemia treated with arsenic trioxide. J Clin Oncol 18:2620–2625
Hussein MA, Elson P, Reed J et al (2003) Arsenic trioxide (Trisenox™), ascorbic acid (AA), and dexamethasone (Dex) pulses-TAD for relapsed refractory progressive multiple myeloma patients. Hematol J 4:S257
Bahlis NJ, McCafferty-Grad J, Jordan-McMurry I et al (2002) Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin Cancer Res 8:3658–3668
Munshi NC, Desikan R, Zangari M (1999) Marked anti-tumor effect of arsenic trioxide (As2O3) in high risk refractory multiple myeloma. Blood 94:123a
Munshi NC, Hideshima T, Chauhan D, Richardson P, Anderson KC (2002) Novel biologically based therapies for multiple myeloma. Int J Hematol 76:340–341
Munshi NC, Tricot G, Desikan R et al (2002) Clinical activity of arsenic trioxide for the treatment of multiple myeloma. Leukemia 16:1835–1837. doi:10.1038/sj.leu.2402599
Donelli A, Chiodino C, Panissidi T, Roncaglia R, Torelli G (2000) Might arsenic trioxide be useful in the treatment of advanced myelodysplastic syndromes? Haematologica 85:1002–1003
Cheson BD, Zwiebel JA, Dancey J, Murgo A (2000) Novel therapeutic agents for the treatment of myelodysplastic syndromes. Semin Oncol 27:560–577
List A, Beran M, DiPersio J et al (2003) Opportunities for Trisenox (arsenic trioxide) in the treatment of myelodysplastic syndromes. Leukemia 17:1499–1507. doi:10.1038/sj.leu.2403021
Vuky J, Yu R, Schwartz L, Motzer RJ (2002) Phase II trial of arsenic trioxide in patients with metastatic renal cell carcinoma. Invest New Drugs 20:327–330. doi:10.1023/A:1016270206374
Lecureur V, Lagadic-Gossmann D, Fardel O (2002) Potassium antimonyl tartrate induces reactive oxygen species-related apoptosis in human myeloid leukemic HL60 cells. Int J Oncol 20:1071–1076
Mann KK, Davison K, Colombo M et al (2006) Antimony trioxide-induced apoptosis is dependent on SEK1/JNK signaling. Toxicol Lett 160:158–170. doi:10.1016/j.toxlet.2005.06.017
Gartenhaus RB, Prachand SN, Paniaqua M, Li Y, Gordon LI (2002) Arsenic trioxide cytotoxicity in steroid and chemotherapy-resistant myeloma cell lines: enhancement of apoptosis by manipulation of cellular redox state. Clin Cancer Res 8:566–572
Chouchane S, Snow ET (2001) In vitro effect of arsenical compounds on glutathione-related enzymes. Chem Res Toxicol 14:517–522. doi:10.1021/tx000123x
Larochette N, Decaudin D, Jacotot E et al (1999) Arsenite induces apoptosis via a direct effect on the mitochondrial permeability transition pore. Exp Cell Res 249:413–421. doi:10.1006/excr.1999.4519
Griffith OW (1982) Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem 257:13704–13712
Dai J, Weinberg RS, Waxman S, Jing Y (1999) Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood 93:268–277
Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S (1999) Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood 94:2102–2111
Chattopadhyay S, Ghosh S, Debnath J, Ghosh D (2001) Protection of sodium arsenite-induced ovarian toxicity by coadministration of l-ascorbate (vitamin C) in mature Wistar strain rat. Arch Environ Contam Toxicol 41:83–89. doi:10.1007/s002440010223
Block G, Henson DE, Levine M (1991) Vitamin C: a new look. Ann Intern Med 114:909–910
Bijur GN, Ariza ME, Hitchcock CL, Williams MV (1997) Antimutagenic and promutagenic activity of ascorbic acid during oxidative stress. Environ Mol Mutagen 30:339–345. doi:10.1002/(SICI) 1098-2280(1997) 30:3<339::AID-EM13>3.0.CO;2-E
Sakagami H, Satoh K (1997) Modulating factors of radical intensity and cytotoxic activity of ascorbate. Anticancer Res 17:3513–3520 Review
Subbarayan PR, Lima M, Ardalan B (2007) Arsenic trioxide/ascorbic acid therapy in patients with refractory metastatic colorectal carcinoma: a clinical experience. Acta Oncol 46:557–561. doi:10.1080/02841860601042456
Berenson JR, Matous J, Swift RA, Mapes R, Morrison B, Yeh HS (2007) A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin Cancer Res 13:1762–1768. doi:10.1158/1078-0432.CCR-06-1812
Wu KL, Beksac M, van Droogenbroeck J, Amadori S, Zweegman S, Sonneveld P (2006) Phase II multicenter study of arsenic trioxide, ascorbic acid and dexamethasone in patients with relapsed or refractory multiple myeloma. Haematologica 91:1722–1723
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lösler, S., Schlief, S., Kneifel, C. et al. Antimony-trioxide- and arsenic-trioxide-induced apoptosis in myelogenic and lymphatic cell lines, recruitment of caspases, and loss of mitochondrial membrane potential are enhanced by modulators of the cellular glutathione redox system. Ann Hematol 88, 1047–1058 (2009). https://doi.org/10.1007/s00277-009-0736-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-009-0736-4