Abstract
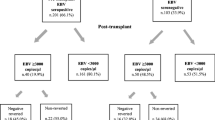
Posttransplant lymphoproliferative disease (PTLD) is closely linked to primary Epstein–Barr virus (EBV) infection. A defect of EBV specific cellular immunity is postulated to play a pivotal role in the etiology of PTLD, but there is some debate as to whether EBV load in the peripheral blood of transplant patients predicts onset of PTLD or relapse after treatment. The current prospective, single-center study was undertaken to investigate the impact of therapy on EBV load in adult patients with PTLD. Fifteen patients with PTLD after solid organ transplantation were included and of these, seven had EBV-associated PTLD. All 15 patients received Rituximab as primary therapy. In cases of treatment failure or relapse after Rituximab treatment, patients received polychemotherapy according to the cyclophosphamide, vincristine, doxorubicin, and prednisone regimen. At onset of PTLD, the median EBV load in the peripheral blood of patients was higher in EBV-associated PTLD than PTLD with no associated EBV infection. After Rituximab therapy, four of seven patients with EBV-associated PTLD achieved long-lasting complete remissions. However, in two of these patients, EBV load increased to reach levels as high as those recorded at onset of PTLD. Another patient showed a dramatic decline of EBV load after the first dose of Rituximab while suffering from progressive disease. The other patient relapsed after Rituximab monotherapy, but his viral load stayed low. In total, discordance in EBV load and clinical course was observed in five of the seven patients with EBV-associated PTLD. We conclude that in adult patients with PTLD, EBV load does not correlate with treatment response and is not suitable as a predictive marker for PTLD relapse.


Similar content being viewed by others
References
Babcock GJ, Decker LL, Freeman RB, Thorley-Lawson DA (1999) Epstein–Barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J Exp Med 190:567–576
Choquet S, Leblond V, Herbrecht R, Socie G, Stoppa AM, Vandenberghe P, Fischer A, Morschhauser F, Salles G, Feremans W, Vilmer E, Peraldi MN, Lang P, Lebranchu Y, Oksenhendler E, Garnier JL, Lamy T, Jaccard A, Ferrant A, Offner F, Hermine O, Moreau A, Fafi-Kremer S, Morand P, Chatenoud L, Berriot-Varoqueaux N, Bergougnoux L, Milpied N (2005) Efficacy and safety of Rituximab in B-cell posttransplant lymphoproliferative disorders: results of a prospective multicentre phase II study. Blood: (Epub ahead of print)
Dotti G, Fiocchi R, Motta T, Gamba A, Gotti E, Gridelli B, Borleri G, Manzoni C, Viero P, Remuzzi G, Barbui T, Rambaldi A (2000) Epstein–Barr virus-negative lymphoproliferate disorders in long-term survivors after heart, kidney, and liver transplant. Transplantation 69:827–833
Fellner MD, Durand K, Correa M, Bes D, Alonio LV, Teyssie AR, Picconi MA (2003) A semiquantitative PCR method (SQ-PCR) to measure Epstein–Barr virus (EBV) load: its application in transplant patients. J Clin Virol 28:323–330
Green M, Cacciarelli TV, Mazariegos GV, Sigurdsson L, Qu L, Rowe DT, Reyes J (1998) Serial measurement of Epstein–Barr viral load in peripheral blood in pediatric liver transplant recipients during treatment for posttransplant lymphoproliferative disease. Transplantation 66:1641–1644
Harris NL, Ferry JA, Swerdlow SH (1997) Posttransplant lymphoproliferative disorders: summary of Society for Hematopathology Workshop. Semin Diagn Pathol 14:8–14
Ibrahim AI, Obeid MT, Jouma MJ, Moasis GA, Al-Richane WL, Kindermann I, Boehm M, Roemer K, Mueller-Lantzsch N, Gartner BC (2005) Detection of herpes simplex virus, cytomegalovirus and Epstein–Barr virus DNA in atherosclerotic plaques and in unaffected bypass grafts. J Clin Virol 32:29–32
Kenagy DN, Schlesinger Y, Weck K, Ritter JH, Gaudreault-Keener MM, Storch GA (1995) Epstein–Barr virus DNA in peripheral blood leukocytes of patients with posttransplant lymphoproliferative disease. Transplantation 60:547–554
Kogan DL, Burroughs M, Emre S, Fishbein T, Moscona A, Ramson C, Shneider BL (1999) Prospective longitudinal analysis of quantitative Epstein–Barr virus polymerase chain reaction in pediatric liver transplant recipients. Transplantation 67:1068–1070
Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, Levy R (1994) Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood 84:2457–2466
Matsukura T, Yokoi A, Egawa H, Kudo T, Kawashima M, Hirata Y, Tanaka H, Kagajo K, Wada H, Tanaka K (2002) Significance of serial real-time PCR monitoring of EBV genome load in living donor liver transplantation. Clin Transplant 16:107–112
Oertel SH, Verschuuren E, Reinke P, Zeidler K, Papp-Vary M, Babel N, Trappe RU, Jonas S, Hummel M, Anagnostopoulos I, Dorken B, Riess HB (2005) Effect of anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD). Am J Transplant 5:2901–2906
Orentas RJ, Schauer DW Jr, Ellis FW, Walczak J, Casper JT, Margolis DA (2003) Monitoring and modulation of Epstein–Barr virus loads in pediatric transplant patients. Pediatr Transplant 7:305–314
Penn I, Hammond W, Brettschneider L, Starzl TE (1969) Malignant lymphomas in transplantation patients. Transplant Proc 1:106–112
Poirel HA, Bernheim A, Schneider A, Meddeb M, Choquet S, Leblond V, Charlotte F, Davi F, Canioni D, Macintyre E, Mamzer-Bruneel MF, Hirsch I, Hermine O, Martin A, Cornillet-Lefebvre P, Patey M, Toupance O, Kemeny JL, Deteix P, Raphael M (2005) Characteristic pattern of chromosomal imbalances in posttransplantation lymphoproliferative disorders: correlation with histopathological subcategories and EBV status. Transplantation 80:176–184
Riddler SA, Breinig MC, McKnight JL (1994) Increased levels of circulating Epstein–Barr virus (EBV)-infected lymphocytes and decreased EBV nuclear antigen antibody responses are associated with the development of posttransplant lymphoproliferative disease in solid-organ transplant recipients. Blood 84:972–984
Rowe DT, Qu L, Reyes J, Jabbour N, Yunis E, Putnam P, Todo S, Green M (1997) Use of quantitative competitive PCR to measure Epstein–Barr virus genome load in the peripheral blood of pediatric transplant patients with lymphoproliferative disorders. J Clin Microbiol 35:1612–1615
Rowe DT, Webber S, Schauer EM, Reyes J, Green M (2001) Epstein–Barr virus load monitoring: its role in the prevention and management of post-transplant lymphoproliferative disease. Transpl Infect Dis 3:79–87
Savoldo B, Rooney CM, Quiros-Tejeira RE, Caldwell Y, Wagner HJ, Lee T, Finegold MJ, Dotti G, Heslop HE, Goss JA (2005) Cellular immunity to Epstein–Barr virus in liver transplant recipients treated with Rituximab for post-transplant lymphoproliferative disease. Am J Transplant 5:566–572
Scheenstra R, Verschuuren EA, de Haan A, Slooff MJ, The TH, Bijleveld CM, Verkade HJ (2004) The value of prospective monitoring of Epstein–Barr virus DNA in blood samples of pediatric liver transplant recipients. Transpl Infect Dis 6:15–22
Stevens SJ, Verschuuren EA, Pronk I, van Der Bij W, Harmsen MC, The TH, Meijer CJ, van Den Brule AJ, Middeldorp JM (2001) Frequent monitoring of Epstein–Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood 97:1165–1171
Vajro P, Lucariello S, Migliaro F, Sokal E, Gridelli B, Vegnente A, Iorio R, Smets F, Quinto I, Scala G (2000) Predictive value of Epstein–Barr virus genome copy number and BZLF1 expression in blood lymphocytes of transplant recipients at risk for lymphoproliferative disease. J Infect Dis 181:2050–2054
Wadowsky RM, Laus S, Green M, Webber SA, Rowe D (2003) Measurement of Epstein–Barr virus DNA loads in whole blood and plasma by TaqMan PCR and in peripheral blood lymphocytes by competitive PCR. J Clin Microbiol 41:5245–5249
Wagner HJ, Cheng YC, Huls MH, Gee AP, Kuehnle I, Krance RA, Brenner MK, Rooney CM, Heslop HE (2004) Prompt versus preemptive intervention for EBV lymphoproliferative disease. Blood 103:3979–3981
Wagner HJ, Fischer L, Jabs WJ, Holbe M, Pethig K, Bucsky P (2002) Longitudinal analysis of Epstein–Barr viral load in plasma and peripheral blood mononuclear cells of transplanted patients by real-time polymerase chain reaction. Transplantation 74:656–664
Yancoski J, Danielian S, Ibanez J, Turconi A, Cuarterolo M, Zelazko M, Niesters Hubert GM (2004) Quantification of Epstein–Barr virus load in Argentinean transplant recipients using real-time PCR. J Clin Virol 31:58–65
Yang J, Tao Q, Flinn IW, Murray PG, Post LE, Ma H, Piantadosi S, Caligiuri MA, Ambinder RF (2000) Characterization of Epstein–Barr virus-infected B cells in patients with posttransplantation lymphoproliferative disease: disappearance after Rituximab therapy does not predict clinical response. Blood 96:4055–4063
Young L, Alfieri C, Hennessy K, Evans H, O’Hara C, Anderson KC, Ritz J, Shapiro RS, Rickinson A, Kieff E et al (1989) Expression of Epstein–Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med 321:1080–1085
Acknowledgements
Stephan Oertel and Ralf Trappe contributed equally in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oertel, S., Trappe, R.U., Zeidler, K. et al. Epstein–Barr viral load in whole blood of adults with posttransplant lymphoproliferative disorder after solid organ transplantation does not correlate with clinical course. Ann Hematol 85, 478–484 (2006). https://doi.org/10.1007/s00277-006-0109-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-006-0109-1