Abstract
Purpose
To histologically describe a direct contact (the so-called dehiscence) of the optic nerve (ON) and/or internal carotid artery (ICA) to the mucosa of posterior paranasal sinuses represented by the sphenoid sinus (SS).
Methods
Observations of histological sections of unilateral or bilateral skull bases (parasellar area and orbital apex) from 22 elderly cadavers were made.
Results
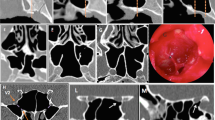
A bony septum was less than 300 µm between the SS and ICA and 200 µm between the SS and optic nerve. Parts of the septa were sometimes absent due to fragmentation and holes of the bony lamella (2/22 facing the ICA; 4 facing the ICA in combination with an absent bony septum facing the nerve). In these dehiscence sites, the SS submucosal tissue attached to a thick sheath (50–100 µm in thickness) enclosing the optic nerve and ophthalmic artery and/or the ICA adventitia (50–200 µm in thickness). The ICA sometimes contained a sclerotic plaque that attached to or even protruded into the SS. With or without dehiscence, the SS mucosa was always thin (50–100 µm in thickness) and accompanied no mononuclear cellular infiltration or tumor.
Conclusions
A thin bony septum of the optic nerve or ICA had been notable as a danger point during surgery, but even a 0.05-mm-thick bone lamella might be an effective barrier against cellular infiltration or bacterial invasion from the SS. Fragmentation and holes of the bony lamella in 4 cadavers might allow cellular invasion to the optic nerve. Accordingly, unknown immunological cross talks might occur to cause demyelination.





Similar content being viewed by others
References
Akdemir G, Tekdemir I, Altin L (2004) Transethmoidal approach to the optic canal: surgical and radiological microanatomy. Surg Neurol 62:268–274
Ali IK, Sansare K, Karjodkar F et al (2020) Imaging analysis of Onodi cells on cone-beam computed tomography. Int Arch Otorhinolaryngol 24:e319–e322
da Silva APB, Silva RBM, Goi LD et al (2020) Experimental models of neuroimmunological disorders: a review. Front Neurol 11:389
Degaga T, Zenebe AM, Wirtu AT et al (2020) Anatomographic variants of sphenoid sinus in Ethiopian population. Diagnostics 10:970
DeLano M, Fun F, Zinreich S (1996) Relationship of the optic nerve to the posterior paranasal sinuses: a CT anatomic study. Am J Neuroradiol 122:293–294
Duan T, Verkman AS (2020) Experimental animal models of aquaporin-4-IgG seropositive neuromyelitis optica spectrum disorders: progress and shortcomings. Brain Pathol 30:13–25
Fujii K, Chambers SM, Rhoton AL (1979) Neurovascular relationships of the sphenoid sinus. J Neurosurg 50:31–39
Gagliardi F, Donofrio CA, Spina A et al (2016) Endoscope-assisted transmaxillosphenoidal approach to the sellar and parasellar regions: an anatomic study. World Neurosurg 95:246–252
Gay D, Dick G, Upton G (1979) Multiple sclerosis associated with sinusitis: case-controlled study in general practice. Lancet 8485:815–819
Graber DJ, Levy M, Kerr D et al (2008) Neuromyelitis optica pathogenesis and aquaporin 4. J Neuroinflammation 5:22
Hosemann W, Draf C (2013) Danger points, complications and medio-legal aspects in endoscopic sinus surgery. GMS Curr Top Otorhinolaryngol Head Neck Surg. https://doi.org/10.3205/cto000098
Itagi RM, Adiga CP, Kalenahalli K et al (2017) Optic nerve canal relation to posterior paranasal sinuses in Indian ethnics: review and objectice classification. J Clin Diagn Res 11:TC01–TC03
Johnson DW, Hopkins RJ, Hanafee WN et al (1985) The unprotected parasphenoidal carotid artery studies by high-resolution computed tomography. Radiology 155:137–141
Kainz J, Stammberger H (1991) Gefahrenpunkte der hinteren Rhinobasis: Anatomische, histologische und endoskopische Befunde. Laryngorhinootologie 70:479–486
Kennedy DW, Zinreich SJ, Hassab MH (1990) The internal carotid artery as it relates to endonasal sphenoethmoidectomy. Am J Rhinol 4:7–12
Lang S, Michael Brainin, Neuhold A et al (1991) Multiple sclerosis associated with sinusitis: a case-controlled MRI study. Proceedings of the XIV symposium neuroradiologicum. pp 121–122
Lennon VA, Kryzer TJ, Pittock SJ et al (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202:473–477
Locatelli M, Di Cristofori A, Draghi R et al (1991) Multiple sclerosis associated with sinusitis: a case-controlled MRI study. In: du Boulay G, Molyneux A, Moseley I (eds) Proceedings of the XIV symposium neuroradiologicum. Springer, Berlin.
Lu Y, Pan J, Qi S, Shi J et al (2011) Pneumatization of the sphenoid sinus in Chinese: the differences from Caucasian and its application in the extended transsphenoidal approach. J Anat 219:132–142
Ozturan O, Yenigun A, Degirmeci N et al (2013) Co-existence of the Onodi cell with the variation of perisphenoidal structures. Eur Arch Otorhinolaryngol 270:2057–2063
Pandit L, Cox LM, Malli C et al (2021) Clostridium bolteae elevated in neuromyelitis optica spectrum disorder in India and shares sequence similarity with AQP4. Neurol Neuroimmunol Neuroinflamm 8:e907
Papadopoulos MC, Bennett JL, Verkman AS (2014) Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurol 10:493–506
Papadopoulos MC, Verkman AS (2012) Aquaporin 4 and neuromyelitis optica. Lancet Neurol 11:535–544
Rahmati A, Ghafari R, AnjomShoa M (2016) Normal variations of sphenoid sinus and the adjacent structures detected in cone beam computed tomography. J Dent 17:32–37
Ratelade J, Verkman AS (2012) Neuromyelitis optica: aquaporin-4 based pathogenesis mechanisms and new therapies. Int J Biochem Cell Biol 44:1519–1530
Ren Z, Wang Y, Duan T et al (2012) Cross-immunoreactivity between bacterial aquaporin-Z and human aquaporin-4: potential relevance to neuromyelitis optica. J Immunol 189:4602–4611
Srikajon J, Siritho S, Ngamsombat C et al (2018) Differences in clinical features between optic neuritis in neuromyelitis optica spectrum disorders and in multiple sclerosis. Mult Scler J 4:1–12
Suzuki H, Oku H, Horie T et al (2014) Changes in expression of aquaporin-4 and aquaporin-9 in optic nerve after crushing in rats. PLoS One 9:e114694
Winter A, Chwalisz B (2020) MRI characteristics of NMO, MOG and MS related optic neuritis. Semin Ophthalmol 35:333–342
Zhang H, Liu X, Cheng Y et al (2013) A new method of locating the optic canal based on structures in sella region: computed tomography study. J Craniofac Surg 24:1011–1015
Acknowledgements
The authors wish to sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially improve patient care and increase overall knowledge for the benefit of all mankind. These donors and their families deserve and have our highest gratitude. This work was supported by JSPS KAKENHI grants (Grant numbers: no. JP20K09895 [S.A.], and JP20K10191 [M.Y.]) and by the Tokyo Dental College Research Branding project.
Funding
This work was supported by JSPS KAKENHI grants (Grant numbers: JP20K09895 [S.A.] and JP20K10191 ([M.Y.]) and by the Tokyo Dental College Research Branding Project.
Author information
Authors and Affiliations
Contributions
GM designed the study. KHC, TM, JS, JI, KH performed the experiments. MY, KK analysed the results. MY, GM, and SA prepared the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cho, K.H., Machida, T., Yamamoto, M. et al. Lost or fragmented bony septum of the optic canal facing the sphenoid sinus: a histological study using elderly donated cadavers. Surg Radiol Anat 44, 511–519 (2022). https://doi.org/10.1007/s00276-022-02910-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-022-02910-1



