Abstract
Purpose
The study aimed to explore hyperintense areas in the cisternal segments of the cranial nerves using magnetic resonance imaging (MRI).
Methods
Seventy outpatients underwent thin-sliced, coronal constructive interference steady-state (CISS) sequence and sagittal T2-weighted MRI following conventional MRI examination.
Results
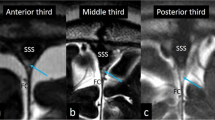
With the coronal CISS sequence, hyperintense areas were located in the central parts of the olfactory bulbs in 65.7% of patients. For the intracranial optic nerve and optic chiasm, hyperintense areas were detected in 98.6% of the CISS sequences and 100% of the T2-weighted images. In the optic tract, hyperintense areas were detected in 51.4% of cases. In 35% of the patients who underwent the CISS sequence, the intracranial optic nerves were considerably compressed by the internal carotid and anterior cerebral arteries, with hyperintense areas similar to those in patients without vascular compression. Hyperintense areas of the cisternal segments of the oculomotor nerve and trigeminal root were identified in 52.9% and 87.1% of the patients, respectively.
Conclusions
The hyperintense areas found within the cisternal segments of the cranial nerves delineated on the coronal CISS sequence and sagittal T2-weighted imaging may indicate the intracranial part of the glymphatic pathway through the cranial nerves. The cranial nerves may function as part of the glymphatic pathway.







Similar content being viewed by others
References
Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J (2019) The glymphatic system and waste clearance with brain aging: a review. Gerontology 65:106–119
Chen L, Elias G, Yostos MP, Stimec B, Fasel J, Murphy K (2015) Pathways of cerebrospinal fluid outflow: a deeper understanding of resorption. Neuroradiology 57:139–147
Eide PK, Ringstad G (2021) Cerebrospinal fluid egress to human parasagittal dura and the impact of sleep deprivation. Brain Res 1772:147669
Fahmy LM, Chen Y, Xuan S, Haacke EM, Hu J, Jiang Q (2021) All central nervous system neuro- and vascular-communication channels are surrounded with cerebrospinal fluid. Front Neurol 12:614636
Jacobsen HH, Ringstad G, Jørstad ØK, Moe MC, Sandell T, Eide PK (2019) The human visual pathway communicates directly with the subarachnoid space. Invest Ophthalmol Vis Sci 60:2773–2780
Jacobsen HH, Sandell T, Jørstad ØK, Moe MC, Ringstad G, Eide PK (2020) In vivo evidence for impaired glymphatic function in the visual pathway of patients with normal pressure hydrocephalus. Invest Ophthalmol Vis Sci 61:24
Killer HE, Miller NR, Flammer J, Meyer P, Weinreb RN, Remonda L, Jaggi GP (2012) Cerebrospinal fluid exchange in the optic nerve in normal-tension glaucoma. Br J Ophthalmol 96:544–548
Klostranec JM, Vucevic D, Bhatia KD, Kortman HGJ, Krings T, Murphy KP, terBrugge KG, Mikulis DJ (2021) Current concepts in intracranial fluid transport and the glymphatic system: Part II-imaging techniques and clinical applications. Radiology 301:516–532
Liang C, Du X, Xu J, Wu L, Liu C, Wang X, Wang H, Yu F (2010) MR imaging of the cisternal segment of the posterior group of cranial nerves: neurovascular relationships and abnormal changes. Eur J Radiol 75:57–63
Mathieu E, Gupta N, Ahari A, Zhou X, Hanna J, Yücel YH (2017) Evidence for cerebrospinal fluid entry into the optic nerve via a glymphatic pathway. Invest Ophthalmol Vis Sci 58:4784–4791
Mezey É, Szalayova I, Hogden CT, Brady A, Dósa Á, Sótonyi P, Palkovits M (2021) An immunohistochemical study of lymphatic elements in the human brain. Proc Natl Acad Sci USA 118:e2002574118
Proulx ST (2021) Cerebrospinal fluid outflow: a review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci 78:2429–2457
Tsutsumi S, Ogino I, Miyajima M, Nakamura M, Yasumoto Y, Arai H, Ito M (2014) Cranial arachnoid protrusions and contiguous diploic veins in CSF drainage. AJNR Am J Neuroradiol 35:1735–1739
Tsutsumi S, Ono H, Yasumoto Y (2017) Visualization of the olfactory nerve using constructive interference in steady state magnetic resonance imaging. Surg Radiol Anat 39:315–321
Tsutsumi S, Fernandez-Miranda JC, Ono H, Yasumoto Y (2017) The cisternal segments of the oculomotor nerve: a magnetic resonance imaging study. Surg Radiol Anat 39:323–331
Tsutsumi S, Ono H, Ishii H (2021) Hyperintense areas in the intraorbital optic nerve evaluated by T2-weighted magnetic resonance imaging: a glymphatic pathway? Surg Radiol Anat 43:1273–1278
Tsutsumi S, Ono H, Ishii H (2021) Trochlear cistern of the cavernous sinus: an anatomical study using magnetic resonance imaging. Surg Radiol Anat 43:1279–1284
Walter BA, Valera VA, Takahashi S, Ushiki T (2006) The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol Appl Neurobiol 32:388–396
Weller RO, Kida S, Zhang ET (1992) Pathways of fluid drainage from the brain—morphological aspects and immunological significance in rat and man. Brain Pathol 2:277–284
Yankova G, Bogomyakova O, Tulupov A (2021) The glymphatic system and meningeal lymphatics of the brain: new understanding of brain clearance. Rev Neurosci 32:693–705
Funding
This study did not receive grant funding.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally to the study.
Corresponding author
Ethics declarations
Ethical approval
All procedures in this study were performed in accordance with the ethical standards of the institutional and/or national research committee, in addition to the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflict of interest
The authors have no conflicts of interest to declare regarding the materials or methods used or the findings presented in this study.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsutsumi, S., Ono, H. & Ishii, H. Hyperintense areas in the cisternal segments of the cranial nerves: a magnetic resonance imaging study. Surg Radiol Anat 44, 503–509 (2022). https://doi.org/10.1007/s00276-022-02902-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-022-02902-1