Abstract
Knowledge of the morphological variations within the abdominal cavity is significant for all medical practitioners planning surgery. This report presents the rare origin of a common trunk for the right inferior phrenic artery, and superior and inferior suprarenal artery from the right renal artery. An accessory hepatic artery was found, which served as a branch of the right inferior phrenic artery. The diameter of the common trunk was 3.95 mm, and the diameters of the inferior and superior suprarenal arteries were 1.84 and 1.36 mm, respectively. The diameter of the right inferior phrenic artery was 2.55 mm. Both the embryological background and the potential clinical significance of this morphological variation are discussed. Knowledge of this common trunk and the occurrence of the accessory right hepatic artery may be of significance in diagnostic and surgical procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The morphological variability existing between unpaired branches of the abdominal aorta has attracted the attention of anatomists, surgeons and radiologists due to its significance in a range of clinical procedures, including surgery of aneurysms or radiological transarterial chemoembolization procedures for tumours [19, 25, 26, 30].
A great deal of variation is seen in the origins of the inferior phrenic arteries (IPA), i.e. the right inferior phrenic artery (RIPA) and the left inferior phrenic artery (LIPA); however, they generally originate from the abdominal aorta or celiac trunk [1, 4, 10, 14,15,16, 21, 29, 32, 33], and are known to supply the diaphragm, oesophagus, stomach, liver, adrenal glands and retroperitoneum. The RIPA and LIPA may arise separately from the lateral aspect of the abdominal aorta, immediately above the celiac trunk, or by a common trunk. Some other variants may derive from the celiac trunk, the suprarenal, hepatic, left gastric, renal or superior mesenteric artery [1, 4, 10, 11, 14,15,16, 21, 25, 29, 32, 33].
The occurrence of accessory hepatic arteries (AHA), the left accessory hepatic artery (LAHA) and the right accessory hepatic artery (RAHA) have been described previously [12, 22,23,24,25, 30, 35].
Adrenal arteries (AA) supply the adrenal gland, and consist of three arteries: the superior suprarenal artery (SSA), the middle suprarenal artery (MSA) and the inferior suprarenal artery (ISA). The SSA arises from the LIPA, the MSA arises from the aorta between the IPA and the renal artery (RA), while the ISA derives from the RA; however, they may show variations in their origins [2, 7,8,9, 20, 21].
This case report describes a rare variant origin of a common trunk for the right inferior phrenic artery (which gives the right accessory hepatic artery) and the superior and inferior suprarenal artery from the right renal artery. Our findings highlight the importance of knowledge of the arterial supply in the abdominal cavity, and these findings are significant for radiologists, anatomists and surgeons specializing in the hepato-bilary and pancreatic areas.
Case report
The cadaver of a 64-year-old man was subjected to routine anatomical dissection for research and teaching purposes at the Department of Normal and Clinical Anatomy of the Medical University of Lodz. The dissection was performed in the abdominal cavity. A careful resection was performed of the interrupting tissues, where a common trunk of the right inferior phrenic artery, and superior and inferior suprarenal artery originated from the right renal artery, which originated from the anterior side of the abdominal aorta. The further course of the inferior right inferior phrenic artery gave rise to the right accessory hepatic artery (Figs. 1, 2). The right middle suprarenal artery was absent (Figs. 1, 2).
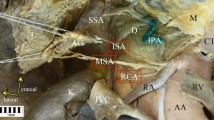
Origin of a common trunk for the right inferior phrenic artery (which gives rise to the right accessory hepatic artery) and inferior and superior suprarenal artery. White arrowheads present the common trunk for the right inferior phrenic artery, and inferior and superior suprarenal artery. AO abdominal aorta, CT celiac trunk, SMA superior mesenteric artery, RIPA right inferior phrenic artery, RAHA right accessory hepatic artery, SSA superior suprarenal artery, ISA inferior suprarenal artery, RA renal artery
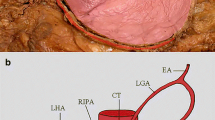
Schema of a common trunk for the right inferior phrenic artery (which gives the right accessory hepatic artery) and inferior and superior suprarenal artery. White arrowheads present the common trunk for the right inferior phrenic artery, and inferior and superior suprarenal artery. AO abdominal aorta, SMA superior mesenteric artery, RIPA right inferior phrenic artery, RAHA right accessory hepatic artery, AG adrenal gland, SSA superior suprarenal artery, ISA inferior suprarenal artery, RA renal artery, IVC inferior vena cava, K kidney, L liver, D diaphragm, CT celiac trunk, CHA common hepatic artery, SA splenic artery
The arteries were measured using digital photographic documentation processed through MultiScanBase 18.03 (Computer Scanning System II, Warsaw, Poland). The value and precision of this method have been confirmed in a previous study [26, 31].
The diameter of the renal artery at its origin from the abdominal aorta was 10.03 mm (the diameter of the coeliac trunk was 12.84 mm, while the diameter of the superior mesenteric artery was 10.17 mm). After 12.53 mm, the right renal artery gave rise to a common trunk of the right inferior phrenic artery, and the superior and inferior suprarenal arteries. The diameter of the common trunk was 3.95 mm, the first branch was the inferior suprarenal artery (diameter 1.84 mm); following this, the course was divided into the superior suprarenal artery (diameter 1.36 mm) and the common trunk of the right inferior phrenic artery and the right accessory hepatic artery. The diameter of the right inferior phrenic artery was 2.55 mm. Furthermore, it was observed that the right hepatic artery branched out: where it did so, the diameter of the right hepatic artery was 1.13 mm and that of the right inferior phrenic artery was 2.11 mm.
Discussion
Knowledge of the vascularization of hepatic vascular variations is significant for the daily practice for surgeons specializing in the hepato-bilary and pancreatic area, and also for general surgeons and radiologists, mainly those involved in interventional radiology. Significant improvements have been made in the surgical and/or radiological treatment of benign and malignant diseases of the liver, pancreas and bile ducts. In laparoscopic surgery, there is a need for accurate descriptions of liver vascularization to avoid iatrogenic vascular changes [25, 26, 30].
An understanding of the extent of vascularization by the IPA is significant because, apart from the main supply of the diaphragm, it can form collateral circulation. Liver cancers commonly derive their arterial supply from the hepatic arteries, and the RIPA and the LIPA are pathways extrahepatic collateral arteries; they can also supply hepatic malignancies, because they neighbour hepatic segments as they transverse the bare area of the liver [11, 13]. Among the arterial pathways that provide liver cancer, both RIPA and LIPA represent nearly half of the collaterals, with RIPA being the most common [11, 13] and LIPA being the fourth or sixth most common [11, 13]. As a result, both RIPA and LIPA are used during transcatheter arterial chemoembolization of liver tumours, especially those located in the peripheral segments of the liver (1–4) [11, 13].
Variations in the origin of the phrenic arteries are numerous and supplementary phrenic vessels are common [1, 10, 15, 29, 32, 33]. The inferior phrenic arteries arise from a common trunk (55%), from the aorta or the celiac trunk in 18–30% of cases, or as independent branches from these same sources in 62% [32]. Other sources may be the hepatic, left gastric, renal, suprarenal, or superior mesenteric arteries in about 8% of cases [32]. When independent, the right and left phrenics usually arise asymmetrically. The study by Grieg et al. [10] of 848 bodies, the origin of the inferior phrenic is as follows: right and left separately from the celiac trunk, 20.3%; as a common trunk from the aorta, 19.7%; right artery from the aorta, left from the celiac, 14.2%; common trunk from the celiac, 13.6%; separately from the aorta, 13.2%; right from the celiac, left from the aorta, 6.8%; right from the renal, left from the aorta, 3.7%; right from the renal, left from the celiac, 3.5%; right and left from the left gastric, 0.7%; right from left gastric, left from aorta. 0.5%; right from the aorta, left from renal, 0.5%; right from celiac, left from left gastric, 0.5%; right from aorta, left from left gastric, 0.4%; right and left from renal, 0.4%; and all other sources and combinations, 1.9% [10]. Also, in some cases in which the right inferior gastric arose from a renal artery, it was from a superior polar renal artery [10].
More recent studies indicate that the RIPA most commonly originates from the abdominal aorta, as noted by Kimura et al. [14], Basilie et al. [5] and Gürses et al. [11]; however, Loukas et al. [16] and Aslaner et al. [4] report the most common origin of RIPA to be the celiac trunk. The second most frequent type of RIPA origin was found to be the celiac trunk by Kimura et al. [14] and Basilie et al. [5], the abdominal aorta by Loukas et al. [16] and Aslaner et al. [4], and the right renal artery by Gürses et al. [11]. It is worth mentioning that Kimura et al. [14] report the possibility of RIPA originating from the dorsal pancreatic artery, while Aslaner et al. [4] note the possibility of it originating from the common hepatic artery. The difference between the types of origin of the right inferior phrenic artery are shown in Table 1.
The suprarenal arteries demonstrate great morphological variety [2, 7,8,9, 20, 21]. Dutta et al. [8] note that the SSA did not have any variations in origin; this contrasts with our current report, where the SSA origin of the common trunk which originates from the right renal artery. Dutta et al. [8] report the MSA to be absent from the right side in 29% of cases; however, our present findings do not indicate the presence of MSA. While Dutta et al. [8] report variability in the origin of the ISA, i.e. 18% originated from the gonadal arteries and 6% from the right lateral margin of the abdominal aorta, our present study found the ISA to originate from the common trunk.
In a study of 200 cadaveric dissections, Michels [22, 23] describes ten types of hepatic arterial variant. The full classification with its frequency of occurrence is as follows: Type I (normal pattern) − 81%; Type II (a replaced LHA from the left gastric artery) 3%; Type III (a replaced RHA from the superior mesenteric artery) 3.7%; Type IV (replaced RHA and LHA) 0.8%; Type V (an accessory LHA) 3.2%; Type VI (an accessory RHA) 1.6%; Type VII (accessory LHA and RHA) 0.2%; Type VIII (a replaced LHA or RHA with other hepatic artery being an accessory one) 0.35%; Type IX (the hepatic trunk as a branch of the superior mesenteric artery) 1.2% and Type X (common hepatic artery branched from the left gastric artery) 0.04%.
This classification by Michels was later modified by Hiatt et al. [12]. In a study of 1000 livers, Hiatt et al. [12] note the presence of a normal hepatic artery in 757 specimens (75.7%), a LAHA originating from the left gastric artery in 97 specimens (9.7%) and an RAHA originating from the superior mesenteric artery in 106 specimens (10.6%). In 23 cases they also report the presence of a double-replaced pattern, where the right hepatic artery was a branch of the superior mesenteric artery and the left hepatic artery was a branch of the left gastric artery [12]. They also note two variants of origin of the common hepatic artery: as a branch of the superior mesenteric artery in 15 cases (2.3%) and originating directly from the aorta in two cases (1.5%).
López-Andújar et al. [17] classified 12 types of hepatic arterial variations. The full classification is as follows: Type 1 (normal hepatic arterial) 70%; Type 2 (a replaced left hepatic artery arises from the left gastric artery) 9.7%; Type 3 (a replaced right hepatic artery arises from the superior mesenteric artery) 7.8%; Type 4 (presence both replaced right and left hepatic arteries, and the replaced right hepatic artery originated from the superior mesenteric artery, while the left originates from the left gastric artery) 3.1%; Type 5 (the LAHA arises from the left gastric artery) 3.9%; Type 6 (the RAHA originated from the superior mesenteric artery) 0.6%; Type 7 (the LAHA originated from the left gastric artery and RAHA arises from the superior mesenteric artery) 0.6%; Type 8 (replaced left hepatic artery arises from the left gastric artery and the RAHA originated from the superior mesenteric artery) 0.3%; Type 9 (the common hepatic artery arises from the superior mesenteric artery) 2.5%; Type 10 (the common hepatic artery originates from the left gastric artery) 0%; Type 11 (the common hepatic artery arises from the superior mesenteric artery and LAHA is a branch of the left gastric artery) 0.3%; Type 12 (the common hepatic artery arises directly from the aorta) 0.7%.
Noussios et al. [24] report the presence of a normal hepatic arterial anatomy in 81% of examined cases, and 19% were variations on the hepatic arteries: the LAHA was present in 1.6%, while the RAHA in 3.2%; replaced right hepatic artery originated from the superior mesenteric artery in 3.7%, while a replaced left hepatic artery originated from the left gastric artery in 3% of cases. Both replaced right and left hepatic artery were observed in 0.8% of cases [24]. Our previous studies also describe the existence of accessory hepatic arteries, namely five RAHA arising from the celiac trunk, one RAHA branching off the proper hepatic artery, and one RAHA originating from the superior mesenteric artery; it also describes a variant where the LAHA arises from the left gastric artery [25].
Some variants of the AHA were also described as a case report. Bastos-Neves et al. [6] reported a rare anatomical variation of the hepatic arterial supply: a RAHA arising directly from the celiac trunk. Panagouli et al. [27] describe a RAHA originating from the left gastric artery, while Polguj et al. [30] describe a RAHA originating from the common hepatic artery near the celiac trunk, which ran behind the portal vein to the right lobe of the liver. Yamashita et al. [35] describe a RAHA branching from the gastroduodenal artery. Lurie et al. [18] and Paraskevas et al. [28] describe a LAHA originating from the left gastric artery.
The identification of variations and anomalies in the right hepatic artery is not only of value to the anatomist, but also to the surgeon. The RAHA may be injured during resection of the pancreatic head, because the artery is located in close proximity to the portal vein [34]. The presence of replaced right hepatic artery can save live of patients with biliary tract cancer, because they are further away from the bile duct and tendo to save a cancer, allowing the tumour to be excised [34].
The common trunk and the branches off this trunk described in this case report are clinically important: the right inferior phrenic artery may form collateral pathways for liver cancer, thus better facilitating surgical procedures. The presence of the AHA is an important consideration in surgery, because recent literature suggests livers with an AHA are preferred for donor liver transplantation. In addition, Aramaki et al. [3] propose the use of right-sided grafts from donors with an LAHA and a left-sided graft from donors with an RAHA. Knowledge of such anatomical variation is clearly significant in these cases.
Conclusion
In conclusion, although the common trunk of the inferior phrenic artery, superior and inferior suprarenal artery and presence of the right accessory hepatic artery observed in this case are very rare, it might be a highly significant factor in the arterial supply to this region. Preoperative knowledge of such anatomic variants is essential in planning surgical procedure such as liver transplantation or removal of the liver lobe.
References
Adachi B (1928) Das Arteriensystem der Japaner. Verlag der Kaiserlich-Japanischen Universitat zu Kyoto 2:18–71
Anson BJ, Cauldwell EW (1947) The blood supply of the kidney, suprarenal gland, and associated structures. Surg Gynecol Obstet 84:313–320
Aramaki O, Sugawara Y, Kokudo N, Takayama T, Makuuchi M (2006) Branch patch reconstruction in living donor liver transplantation: arterialization of grafts with replaced type arteries. Transplantation 82:1541–1543. https://doi.org/10.1097/01.tp.0000236102.36326.a6
Aslaner R, Pekcevik Y, Sahin H, Toka O (2017) Variations in the origin of inferior phrenic arteries and their relationship to celiac axis variations on CT angiography. Korean J Radiol 18:336–344. https://doi.org/10.3348/kjr.2017.18.2.336
Basile A, Tsetis D, Montineri A, Puleo S, Massa Saluzzo C, Runza G, Coppolino F, Ettorre GC, Patti MT (2008) MDCT anatomic assessment of right inferior phrenic artery origin related to potential supply to hepatocellular carcinoma and its embolization. J Vasc Interv Radiol 31:349–358. https://doi.org/10.1007/s00270-007-9236-x
Bastos-Neves D, da Silva Alves JA, Guedes Dias LG, de Rezende MB, Salvalaggio PR (2016) Right Accessory Hepatic Artery Arising From Celiac Trunk—Case Report of a Variation that Must Be Looked for During Multiorgan Procurement. Transplant Proc. 48:2387–2388. https://doi.org/10.1016/j.transproceed.2016.06.025
Bergman RA, Afifi AK, Miyauchi R (2015) Anatomy atlases: illustrated encyclopedia of human anatomic variation—anatomical variation|radiology anatomy|anatomy atlas. http://www.anatomyatlases.org/AnatomicVariants/AnatomyHP.shtml. Accessed 6 Feb 2017
Dutta S (2010) Suprarenal gland–arterial supply: an embryological basis and applied importance. Rom J Morphol Embryol 51:137–140
Gagnon R (1964) Middle suprarenal arteries in man; “a statistical study of two hundred human adrenal glands’. Rev Can Biol 23:461–467
Grieg H, Anson BJ, Coleman S (1951) The inferior phrenic artery. Types of origin in 850 body-halves and diaphragmatic relationship. Q Bull Northwest Univ Med Sch 25(4):345–350
Gürses İA, Gayretli Ö, Kale A, Öztürk A, Usta A, Şahinoğlu K (2015) Inferior phrenic arteries and their branches, their anatomy and possible clinical importance: an experimental cadaver study. Balkan J Med 32:189–195. https://doi.org/10.5152/balkanmedj.2015.150052
Hiatt JR, Gabbay J, Busuttil RW (1994) Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 220:50–52
Kim H-C, Chung JW, Lee W, Jae HJ, Park JH (2005) Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. Radiographics 25:S25–S39. https://doi.org/10.1148/rg.25si055508
Kimura S, Okazaki M, Higashihara H, Nozaki Y, Haruno M, Urakawa H, Koura S, Shinagawa Y, Nonokuma M (2007) Analysis of the origin of the right inferior phrenic artery in 178 patients with hepatocellular carcinoma treated by chemoembolization via the right inferior phrenic artery. Acta Radiol 48:728–733. https://doi.org/10.1080/02841850701376334
Larcher (1873) Artère diaphragmatique inférieure droite, naissant de la rénale. Lyon Med 12:386
Loukas M, Hullett J, Wagner T (2005) Clinical anatomy of the inferior phrenic artery. Clin Anat 18:357–365. https://doi.org/10.1002/ca.20112
López-Andújar R, Moya A, Montalvá E, Berenguer M, De Juan M, San Juan F, Pareja E, Vila JJ, Orbis F, Prieto M, Mir J (2007) Lessons learned from anatomic variants of the hepatic artery in 1,081 transplanted livers. Liver Transpl 13:1401–1404. https://doi.org/10.1002/lt.21254
Lurie AS (1987) The significance of the variant left accessory hepatic artery in surgery for proximal gastric cancer. Arch Surg 122:725. https://doi.org/10.1001/archsurg.1987.01400180107021
Majos M, Stefańczyk L, Szemraj-Rogucka Z, Elgalal M, de Caro R, Macchi V, Polguj M (2017) Does the type of renal artery anatomic variant determine the diameter of the main vessel supplying a kidney? A study based on CT data with a particular focus on the presence of multiple renal arteries. Surg Radiol Anat 0:1–8. https://doi.org/10.1007/s00276-017-1930-z
Manso JC, DiDio LJA (2000) Anatomical variations of the human suprarenal arteries. Ann Anat 182:483–488. https://doi.org/10.1016/S0940-9602(00)80064-3
Merklin R, Michels N (1958) The variant renal and suprarenal blood supply with data on the inferior phrenic, ureteral and gonadal arteries: a statistical analysis based on 185 dissections and review of the literature. J Int Coll Surg 29:41–76
Michels N (1955) Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. Lippincott, Philadeplphia
Michels NA (1966) Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 112:337–347
Noussios G, Dimitriou I, Chatzis I, Katsourakis A (2017) The main anatomic variations of the hepatic artery and their importance in surgical practice: review of the literature. J Clin Med Res 9:248–252. https://doi.org/10.14740/jocmr2902w
Olewnik A, Wysiadecki G, Polguj M, Jankowski M, Topol M (2017) Types of coeliac trunk branching including accessory hepatic arteries: a new point of view based on cadaveric study. Folia Morphol. https://doi.org/10.5603/FM.a2017.0053
Olewnik Ł, Wysiadecki G, Polguj M, Topol M (2017) A rare anastomosis between the common hepatic artery and the superior mesenteric artery: a case report. Surg Radiol Anat. https://doi.org/10.1007/s00276-017-1859-2
Panagouli E, Venieratos D (2012) Right accessory hepatic artery arising from the left gastric artery: a case report. Rom J Morphol Embryol 52:1143–1145
Paraskevas GK, Raikos A (2011) Multiple aberrant coeliac trunk ramifications. Singapore Med J l 52:e147-9
Pick JW, Anson BJ (1940) The inferior phrenic artery: origin and suprarenal branches. Anat Rec 78:413–427. https://doi.org/10.1002/ar.1090780402
Polguj M, Gabryniak T, Topol M (2010) The right accessory hepatic artery; a case report and review of the literature. Surg Radiol Anat 32:175–179. https://doi.org/10.1007/s00276-009-0536-5
Polguj M, Jȩdrzejewski KS, Topol M (2011) Angioarchitecture of the bovine spermatic cord. J Morphol 272:497–502. https://doi.org/10.1002/jmor.10929
Poynter CWM (1922) Congenital anomalies of the arteries and veins of the human body with bibliography, vol 22, 22nd edn. The University Studies of the University of Nebraska, pp 1–106
Quain R (1844) Anatomy of the arteries of the human body. Taylor and Walton, London
Standring S (2008) Gray’s anatomy: the anatomical basis of clinical practice, 40th edn. Churchill Livingstone Elsevier, New York
Yamashita K, Hashimoto D, Itoyama R, Okabe H, Chikamoto A, Beppu T, Baba H (2015) Accessory right hepatic artery branched from gastroduodenal artery. Surg Case Rep 1:90. https://doi.org/10.1186/s40792-015-0092-7
Acknowledgements
The authors wish to express their gratitude to all those who donated their bodies to medical science.
Funding
There is no funding source.
Author information
Authors and Affiliations
Contributions
ŁO—project development, data collection and management, data analysis and manuscript writing. AW—data collection, data analysis, manuscript editing. MP—data analysis, manuscript editing. MT—data analysis, manuscript editing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Olewnik, Ł., Waśniewska, A., Polguj, M. et al. Rare combined variations of renal, suprarenal, phrenic and accessory hepatic arteries. Surg Radiol Anat 40, 743–748 (2018). https://doi.org/10.1007/s00276-018-2026-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-018-2026-0