Abstract
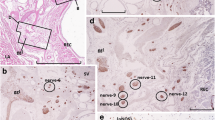
In pelvic surgery, much attention is paid to nerve bundles but not to ganglion cells. Using serial section histology of 14 postmortem-treated hemipelvis (eight males, six females; mean, 79 years old), we examined the population number, distribution, and tyrosine hydroxylase-immunoreactivity (TH-IR; suggesting sympathetic neurons) of extramural pelvic ganglion cells. All pelvic ganglion cells were uniformly sized (25–30 μm) contrasting with small intramural rectal neurons. Abundant ganglion cells (30,000–140,000 unilaterally) existed not only along the pelvic viscera except for the rectum, but also along the hypogastric nerve, pelvic splanchnic nerve, pelvic plexus, and associated branches excluding those within the mesorectum. The intrapelvic ganglion cells outside the sympathetic trunk did not form macroscopically identifiable ganglia, but made small clusters (0.1–2.0 mm of maximum diameter) or were diffusely scattered within nerve bundles. More than half of these cells appeared TH-IR positive, although the positive/negative proportion differed between nerves and specimens. Greater numbers of ganglion cells were found in dorsosuperior sites (e.g., around the seminal vesicle) rather than in ventroinferior sites (e.g., along the urethra) in males, and vice versa in females. However, in total cell numbers, interindividual variations were evident rather than intergender difference. Due to significant interindividual variations in cell number, differences are likely to exist between patients in “resistance” to surgical stresses. We hypothesized that pelvic ganglion cells are liable to be damaged due to drying along the surgical margin, hypoxia in venous bleeding, pressure from surgical retractors, extension stress with taping and excess traction and/or direct injury with electrical scalpels.




Similar content being viewed by others
References
Akita K, Sakamoto H, Sato T (2003) Origins and courses of the nervous branches to the male urethral sphincter. Surg Radiol Anat 25:387–392
Ali M, Johnson IP, Hobson J, Mohammadi B, Khan F (2004) Anatomy of the pelvic plexus and innervation of the prostate gland. Clin Anat 17:123–129
Ball TP Jr, Teickman JMH, Sharkey FE, Rogenes VJ, Adrian EK Jr (1997) Terminal nerve distribution to the urethra and bladder neck: consideration in the management of stress urinary incontinence. J Urol 158:827–829
Boyd-Clark LC, Briggs CA, Galea MP (2004) Segmental degeneration in the cervical spine and associated changes in dorsal root ganglia. Clin Anat 17:468–477
Butler-Manuel SA, Buttery LDK, A’Hern RP, Polak JM, Barton DPJ (2002) Pelvic nerve plexus trauma at radical and simple hysterectomy: a quantitative study of nerve types in the uterine supporting ligaments. J Soc Gynecol Invest 9:47–56
Dail WG, Galindo R, Leyba L, Barba V (1997) Denervation-induced changes in perineuronal plexuses in the major pelvic ganglion of the rat: immunohistochemistry for vasoactive intestinal polypeptide and tyrosine hydroxylase and histochemistry for NADPH-diaphorase. Cell Tissue Res 287:315–324
Diop M, Parratte B, Tatu L, Vuillier F, Brunelle S, Monnier G (2003) Mesorectum: the surgical value of an anatomical approach. Surg Radiol Anat 25:290–304
Fritsch H, Lienenmann A, Brenner E, Ludwikowski B (2004) Clinical anatomy of the pelvic floor. Adv Anat Embryol Cell Biol 175(III–IX):1–64
Hinman F Jr (1993) Atlas of urosurgical anatomy. Saunders, Philadelphia, PA, pp42–45, 209, 444
Honma H, Gross LA, Windebank AJ (2004) Hypoxia-induced apoptosis of dorsal root ganglion neurons is associated with DNA damage recognition and cell cycle disruption in rats. Neurosci Lett 354:95–98
Jen PYP, Dixon JS, Gosling JA (1995) Co-localization of tyrosine hydroxylase, nitric oxide synthetase and neuropeptides in neurons of the human postnatal male pelvic ganglia. Auton Nerv Syst 59:41–50
Kato T, Murakami G, Yabuki Y (2002) Does the cardinal ligament of the uterus contain nerve fibers that should be preserved during nerve-sparing radical hysterectomy. Anat Sci Int 77:161–168
Kepper M, Keast J (1995) Immunohistological properties and spinal connections of pelvic autonomic neurons that innervate the rat prostate gland. Cell Tissue Res 281:33–542
Kinugasa Y, Murakami G, Uchimoto K, Takenaka A, Yajima T, Sugihara K (2006) Operating behind Denonvilliers’ fascia for reliable preservation of urogenital autonomic nerves in total mesorectal excision: a histological study using fresh and fixed cadaveric specimens. Dis Colon Rectum 49:1–9
Kurihara M, Murakami G, Kajiwara M, Taguchi K, Tshukamoto T, Usui T (2004) Lack of the completely circular rhabdosphincter and a distinct circular smooth muscle layer around the proximal urethra in elderly Japanese women. Int Uro Gynecol Pelvic Floor Dysfunct 15:85–94
Li MZ, Masuko S (2001) Target specific organization and neuron types of the dog pelvic ganglia: a retrograde-tracing and immunohistochemical study. Arch Histol Cytol 64:267–280
Maas CP, Moriya Y, Steup WH, Kiebert GM, Kranenberg WM, van de Velde CJH (1998) Radical and nerve-preserving surgery for rectal cancer in The Netherlands: a prospective study on morbidity and functional outcome. Br J Surg 85:92–97
Maurer CA, Z’Graggen K, Renzulli P, Schilling MK, Netzer P, Buchler NW (2001) Total mesorectal excision preserves male genital function compared with conventional rectal cancer surgery. Br J Surg 88:1501–1505
Milley PS, Nichols DH (1969) A correlative investigation of the human rectovaginal septum. Anat Rec 163:443–452
Murakami G, Matsumura H (2003) Anatomy and histology of the intrapelvic autonomic nerves with special reference to the topographical relationship with fascial structures. Urol Surg 16:651–660 (in Japanese)
Nesbakken A, Nygaard K, Bull-Njaa T, Carlsen E, Eri LM (2000) Bladder and sexual dysfunction after mesorectal excision for rectal cancer. Br J Surg 87:206–210
Pethô G, Pórszász R, Peitl B, Szolcsányi J (1999) Spike generation from dorsal roots and cutaneous afferents by hypoxia or hypercapnia in the rat in vivo. Exp Physiol 84:1–15
Pocard M, Zinzindohoue F, Haab F, Caplin S, Parc R, Tiret E (2002) A prospective study of sexual and urinary function before and after total mesorectal excision with autonomic nerve preservation for rectal cancer. Surgery 131:368–372
Purinton PT, Fletcher TF, Bradley WE (1973) Gross and light microscopic features of the pelvic plexus in the rat. Anat Rec 175:697–706
Romanes GJ (1981) Cunningham’s textbook of anatomy, 12th edn. Oxford University Press, Oxford
Sugawara O, Atsuta Y, Iwahara T, Muramoto T, Watakabe M, Takemitsu Y (1996) The effects of mechanical compression and hypoxia on nerve root and dorsal root ganglia: an analysis of ectopic firing using an in vitro model. Spine 21:2089–2094
Taguchi K, Tsukamoto T, Murakami G (1999) Anatomical studies of the autonomic nervous system in the human pelvis by the whole-mount staining method: left-right communicating nerves between bilateral pelvic plexuses. J Urol 161:320–325
Takenaka A, Murakami G, Soga H, Han SH, Arai Y, Fujisawa M (2004) Anatomical analysis of the neurovascular bundle supplying penile cavernous tissue to ensure a reliable nerve graft after radical prostatectomy. J Urol 172:1032–1035
Takenaka A, Kawada T, Murakami G, Hisasue S, Tsukamoto T, Fujisawa M (2005) Interindividual variation in distribution of extramural ganglion cells in the male pelvis: a semi-quantitative and immunohistochemical study concerning nerve-sparing pelvic surgery. Eur J Urol 48:46–52
Takenaka A, Murakami G, Matsubara A, Han SH, Fujisawa M (2005) Variation in course of cavernous nerve with special reference to details of topographic relationships near prostatic apex: histologic study using male cadavers. Urol 65:136–142
Tamakawa M, Murakami G, Takashima K, Kato T, Hareyama M (2003) Fascial structures and autonomic nerves in the female pelvis: a study using macroscopic slices and their corresponding histology. Anat Sci Int 78:228–242
Walsh PC, Donker PJ (1982) Impotence following radical prostatectomy: insights into etiology and prevention. J Urol 128:492–497
Williams PL (1995) Gray’s anatomy, 38th edn. Churchill Livingstone, London, pp1778, 1782, 1874
Acknowledgments
We thank Mr. Seiji Ohtani and Ms. Mami Yamaguchi in the biomedical laboratory center of Sapporo Medical University for their assistance in immunohistochemistry. We are also grateful to Professor Toshihiko Yajima (Department of Oral Anatomy, Health Science University of Hokkaido School of Dentistry) for kind permission of the use of his materials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imai, K., Furuya, K., Kawada, M. et al. Human pelvic extramural ganglion cells: a semiquantitative and immunohistochemical study. Surg Radiol Anat 28, 596–605 (2006). https://doi.org/10.1007/s00276-006-0156-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-006-0156-2