Abstract
Purpose
Antiangiogenic agents have been used for many years as a first-line systemic treatment for advanced HCC. Embolization with cytostatic drugs on the other hand is the first-line treatment for intermediate HCC. The two types of drugs have not been combined for intraarterial delivery yet. The loading and release dynamics and the in vitro effect of their combination are tested in this experimental study.
Materials and Methods
Drug-eluting beads were loaded with doxorubicin, sunitinib and sunitinib analogue piperazine (SAP) alone and with their combinations. Diameter change, loading, release, and effect in cellular proliferation were assessed.
Results
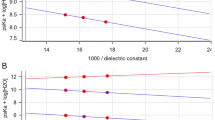
The average microsphere diameter after loading was 473.7 µm (μm) for Doxorubicin, 388.4 μm for Sunitinib, 515.5 μm for SAP, 414.8 μm for the combination Doxorubicin/Sunitinib and 468.8 μm for the combination Doxorubicin /SAP. Drug release in 0.9% NaCl was 10% for Doxorubicin, 49% for Sunitinib, 25% for SAP, 20%/18% for the combination Doxorubicin/Sunitinib, and 18%/23% for the combination Doxorubicin/SAP whereas in human plasma it was 56%, 27%, 13%, 76%/63% and 62%/15%, respectively. The mean concentration of Doxorubicin that led to inhibition of 50% of cellular proliferation in an HCC Huh7 cell line was 163.1 nM (nM), for Sunitinib 10.3 micromolar (μΜ), for SAP 16.7 μΜ, for Doxorubicin/Sunitinib 222.4 nM and for Doxorubicin/SAP 275 nM.
Conclusions
Doxorubicin may be combined with antiangiogenic drugs with satisfactory in vitro loading and release outcomes and effect on cellular lines.
Graphical Abstract








Similar content being viewed by others
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- SAP:
-
Sunitinib Analogue Piperazine
- μM:
-
Micromolar
- nM:
-
Nanomolar
- μm:
-
Micrometres
- nm:
-
Nanometres
- TACE:
-
Transarterial chemoembolization
- TKIs:
-
Tyrosine kinase inhibitors
- HPLC:
-
High-performance liquid chromatography
References
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55.
European Association for Study of Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48(5):599–641.
Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93. https://doi.org/10.1016/j.jhep.2021.11.018.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019.
Llovet JM, Real MI, Montaña X, et al. Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–9.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–42.
Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–81.
Lencioni R, de Baere T, Burrel M, et al. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC bead (DEBDOX): technical recommendations. Cardiovasc Interv Radiol. 2012;35(5):980–5.
Xie ZB, Wang XB, Peng YC, et al. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res. 2015;45:190–200.
Sacco R, Bargellini I, Bertini M, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol JVIR. 2011;22:1545–52.
Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–64.
Malagari K, Pomoni M, Moschouris H, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Interv Radiol. 2012;35:1119–28.
Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Interv Radiol. 2010;33:41–52.
Cersosimo RJ. Systemic targeted and immunotherapy for advanced hepatocellular carcinoma. Am J Health Syst Pharm. 2021;78:187–202.
Contratto M, Wu J. Targeted therapy or immunotherapy? Optimal treatment in hepatocellular carcinoma. World J Gastrointest Oncol. 2018;10:108–14.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73.
Yau T, Park W, Finn RS, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30:v874-V875. https://doi.org/10.1093/annonc/mdz394.029.
Finn S, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905.
A Global Study to Evaluate Transarterial Chemoembolization (TACE) in Combination With Durvalumab and Bevacizumab Therapy in Patients With Locoregional Hepatocellular Carcinoma (EMERALD-1). https://clinicaltrials.gov/ct2/show/NCT03778957.
Nivolumab in combination with TACE/TAE for patients with intermediate stage HCC (TACE-3). https://clinicaltrials.gov/ct2/show/NCT04268888.
Safety and efficacy of Lenvatinib (E7080/MK-7902) With Pembrolizumab (MK-3475) in combination with transarterial chemoembolization (TACE) in participants with incurable/non-metastatic hepatocellular carcinoma (MK-7902-012/E7080-G000-318/LEAP-012). https://clinicaltrials.gov/ct2/show/NCT04246177.
Fuchs K, Bize PE, Dormond O, et al. Drug-eluting beads loaded with antiangiogenic agents for chemoembolization: in vitro sunitinib loading and release and in vivo pharmacokinetics in an animal model. J Vasc Interv Radiol. 2014;25(3):379–87.
Argyros O, Karampelas T, Varela A, et al. Targeting of the breast cancer microenvironment with a potent and linkable oxindole based antiangiogenic small molecule. Oncotarget. 2017;8(23):37250–62.
Jordan O, Denys A, De Baere T, Boulens N, Doelker E. Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol. 2010;21(7):1084–90.
Raoul J-L, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36.
Kang YJ, Lee BC, Kim JK, Yim NY, Kim HO, Cho SB, Jeong YY. Conventional versus small doxorubicin-eluting bead transcatheter arterial chemoembolization for treating barcelona clinic liver cancer stage 0/A hepatocellular carcinoma. Cardiovasc Interv Radiol. 2020;43(1):55–64.
Lewis AL, Gonzalez MV, Lloyd AW, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17(2 Pt 1):335–42.
Abdekhodaie MJ, Wu XY. Drug loading onto ion-exchange microspheres: modeling study and experimental verification. Biomaterials. 2006;27(19):3652–62.
Gonzalez MV, Tang Y, Phillips GJ, et al. Doxorubicin eluting beads-2: methods for evaluating drug elution and in-vitro:in-vivo correlation. J Mater Sci Mater Med. 2008;19(2):767–75.
Al-Abd AM, Aljehani ZK, Gazzaz RW, Fakhri SH, Jabbad AH, Alahdal AM, Torchilin VP. Pharmacokinetic strategies to improve drug penetration and entrapment within solid tumors. J Control Release. 2015;10(219):269–77.
Forster RE, Tang Y, Bowyer C, et al. Development of a combination drug-eluting bead: towards enhanced efficacy for locoregional tumour therapies. Anticancer Drugs. 2012;23(4):355–69.
Ranieri G, Ammendola M, Marech I, et al. Vascular endothelial growth factor and tryptase changes after chemoembolization in hepatocarcinoma patients. World J Gastroenterol. 2015;21(19):6018–25.
Liang B, Zheng CS, Feng GS, et al. Correlation of hypoxia-inducible factor 1alpha with angiogenesis in liver tumors after transcatheter arterial embolization in an animal model. Cardiovasc Interv Radiol. 2010;33(4):806–12.
Gupta S, Kobayashi S, Phongkitkarun S, Broemeling LD, Kan Z. Effect of transcatheter hepatic arterial embolization on angiogenesis in an animal model. Invest Radiol. 2006;41(6):516–21.
Rhee TK, Young JY, Larson AC, et al. Effect of transcatheter arterial embolization on levels of hypoxia-inducible factor-1alpha in rabbit VX2 liver tumors. J Vasc Interv Radiol. 2007;18(5):639–45.
Deudero JJ, Caramelo C, Castellanos MC, et al. Induction of hypoxia-inducible factor-1alpha gene expression by vascular endothelial growth factor. J Biol Chem. 2008;283(17):11435–44.
Chung YH, Han G, Yoon JH, et al. Interim analysis of START: study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. Int J Cancer. 2013;132(10):2448–58.
Fuchs K, Bize PE, Denys A, Borchard G, Jordan O. Sunitinib-eluting beads for chemoembolization: methods for in vitro evaluation of drug release. Int J Pharm. 2015;482(1–2):68–74.
Hagan A, Phillips GJ, Macfarlane WM, Lloyd AW, Czuczman P, Lewis AL. Preparation and characterisation of vandetanib-eluting radiopaque beads for locoregional treatment of hepatic malignancies. Eur J Pharm Sci. 2017;1(101):22–30.
Lee KH, Liapi EA, Cornell C, et al. Doxorubicin-loaded QuadraSphere microspheres: plasma pharmacokinetics and intratumoral drug concentration in an animal model of liver cancer. Cardiovasc Interv Radiol. 2010;33(3):576–82.
Benjamin RS, Riggs CE Jr, Bachur NR. Plasma pharmacokinetics of adriamycin and its metabolites in humans with normal hepatic and renal function. Cancer Res. 1977;37(5):1416–20.
Laubrock N, Hempel G, Schulze-Westhoff P, et al. The stability of doxorubicin and ldarubicin in plasma and whole blood. Chromatographia. 2000;52:9–13.
Riss TL, Moravec RA, Niles AL, et al. Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004.
Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42(9):3858–63.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Informed Consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krokidis, M., Fakitsa, D., Malagari, K. et al. Combination of Doxorubicin and Antiangiogenic Agents in Drug-Eluting Beads: In Vitro Loading and Release Dynamics in View of a Novel Therapeutic Approach for Hepatocellular Carcinoma. Cardiovasc Intervent Radiol (2024). https://doi.org/10.1007/s00270-024-03714-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00270-024-03714-z