Abstract
Objective
To compare the cost-effectiveness of drug-coated balloon angioplasty (DCB) versus plain old balloon angioplasty (POBA) for treatment of arteriovenous fistula (AVF) stenosis.
Methods
A Markov model was created to compare DCB versus POBA for AVF stenosis over a 2-year time horizon from a United States payer’s perspective. Probabilities related to complications, restenosis, retreatment, and all-cause mortality were obtained from published literature. Costs were calculated using Medicare reimbursement rates and data from published cost analyses, inflation-adjusted to 2021. Health outcomes were measured with quality-adjusted life years (QALY). Probabilistic and deterministic sensitivity analyses were performed with a willingness-to-pay threshold of $100,000/QALY.
Results
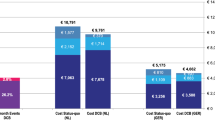
Base case calculation showed better quality-of-life outcomes but increased cost with POBA compared to DCB, with an incremental cost-effectiveness ratio of $27,413/QALY, making POBA the more cost-effective strategy in the base case model. Sensitivity analyses showed that DCB becomes cost-effective if the 24-month mortality rate after DCB is no more than 3.4% higher than that after POBA. In secondary analyses where mortality rates were equalized, DCB was more cost-effective than POBA until its additional cost reached more than $4213 per intervention.
Conclusion
When modeled from a payer’s perspective over 2 years, the cost utility of DCB versus POBA varies with mortality outcomes. POBA is cost-effective if 2-year all-cause mortality after DCB is greater than 3.4% higher than after POBA. If 2-year mortality after DCB is less than 3.4% higher than after POBA, DCB is cost-effective until its additional cost per procedure exceeds $4213 more than POBA.
Level of Evidence IV: Historically Controlled Study.
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.




Similar content being viewed by others
References
Thamer M, Lee TC, Wasse H, et al. Medicare costs associated with arteriovenous fistulas among US Hemodialysis Patients. Am J Kidney Dis. 2018;72:10–8.
Lookstein RA, Haruguchi H, Ouriel K, et al. Drug-coated balloons for dysfunctional dialysis arteriovenous fistulas. N Engl J Med. 2020;383:733–42.
Holden A, Haruguchi H, Suemitsu K, et al. IN.PACT AV access randomized trial: 12-month clinical results demonstrating the sustained treatment effect of drug-coated balloons. J Vasc Interv Radiol 2022;33(8):884–94.e7.
Hu H, Tan Q, Wang J, Liu Y, Yang Y, Zhao J. Drug-coated balloon angioplasty for failing haemodialysis access: meta-analysis of randomized clinical trials. Br J Surg. 2021;108:1293–303.
Han A, Park T, Kim HJ, Min S, Ha J, Min SK. Editor's choice—paclitaxel coated balloon angioplasty vs. plain balloon angioplasty for haemodialysis arteriovenous access stenosis: a systematic review and a time to event meta-analysis of randomised controlled Trials. Eur J Vasc Endovasc Surg 2021; 62:597–609.
Karunanithy N, Robinson EJ, Ahmad F, et al. A multicenter randomized controlled trial indicates that paclitaxel-coated balloons provide no benefit for arteriovenous fistulas. Kidney Int. 2021;100:447–56.
Trerotola SO, Saad TF, Roy-Chaudhury P, Lutonix AVCTI. The Lutonix AV Randomized trial of paclitaxel-coated balloons in arteriovenous fistula stenosis: 2-year results and subgroup analysis. J Vasc Interv Radiol 2020; 31:1–14 e5.
Luo C, Liang M, Liu Y, Zheng D, He Q, Jin J. Paclitaxel coated balloon versus conventional balloon angioplasty in dysfunctional dialysis arteriovenous fistula: a systematic review and meta-analysis of randomized controlled trials. Ren Fail. 2022;44:155–70.
Kitrou PM, Katsanos K, Spiliopoulos S, Karnabatidis D, Siablis D. Drug-eluting versus plain balloon angioplasty for the treatment of failing dialysis access: final results and cost-effectiveness analysis from a prospective randomized controlled trial (NCT01174472). Eur J Radiol. 2015;84:418–23.
Pietzsch JB, Geisler BP, Manda B et al. IN.PACT AV Access trial: economic implications of drug-coated balloon treatment for dysfunctional arteriovenous fistulas. J Vasc Interv Radiol 2022;33(8):895–902.e4
Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103.
Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Public Health. 2022;22:179.
Kuntz K, Sainfort F, Butler M, et al. Decision and simulation modeling in systematic reviews. Rockville (MD), 2013. Report No.: 11(13)-EHC037-EF.
Coskun A, Oosterhuis WP. Statistical distributions commonly used in measurement uncertainty in laboratory medicine. Biochem Med (Zagreb). 2020;30: 010101.
Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–7.
Trerotola SO, Lawson J, Roy-Chaudhury P, Saad TF, Lutonix AVCTI. Drug coated balloon angioplasty in failing av fistulas: a randomized controlled trial. Clin J Am Soc Nephrol. 2018; 13:1215–24.
Yang S, Lok C, Arnold R, Rajan D, Glickman M. Comparison of post-creation procedures and costs between surgical and an endovascular approach to arteriovenous fistula creation. J Vasc Access. 2017;18:8–14.
Nordyke RJ, Reichert H, Bylsma LC, et al. Costs attributable to arteriovenous fistula and arteriovenous graft placements in hemodialysis patients with medicare coverage. Am J Nephrol. 2019;50:320–8.
Rosas SE, Feldman HI. Synthetic vascular hemodialysis access versus native arteriovenous fistula: a cost-utility analysis. Ann Surg. 2012;255:181–6.
Shafrin J, May S, Skrnick M, et al. Use of net monetary benefit analysis to comprehensively understand the value of innovative treatments. Value In Health. 2016;19:A731.
Salisbury AC, Li H, Vilain KR et al. Cost-effectiveness of endovascular femoropopliteal intervention using drug-coated balloons versus standard percutaneous transluminal angioplasty: results from the IN.PACT SFA II Trial. JACC Cardiovasc Interv 2016; 9:2343–52.
Etangsale A, Nunno L, Pineau J, Prognon P, Martelli N. Quality of economic evaluations of drug-coated balloons and drug-eluting stents in peripheral artery disease: a systematic review. Int J Technol Assess Health Care. 2021;37: e79.
Jeger RV, Farah A, Ohlow MA, et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet. 2020;396:1504–10.
Dorenkamp M, Boldt J, Leber AW, et al. Cost-effectiveness of paclitaxel-coated balloon angioplasty in patients with drug-eluting stent restenosis. Clin Cardiol. 2013;36:407–13.
United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
Ferreira ES, Moreira TR, da Silva RG, et al. Survival and analysis of predictors of mortality in patients undergoing replacement renal therapy: a 20-year cohort. BMC Nephrol. 2020;21:502.
Schneider PA, Laird JR, Doros G, et al. Mortality not correlated with paclitaxel exposure: an independent patient-level meta-analysis of a drug-coated balloon. J Am Coll Cardiol. 2019;73:2550–63.
Laird JA, Schneider PA, Jaff MR, et al. Long-term clinical effectiveness of a drug-coated balloon for the treatment of femoropopliteal lesions. Circ Cardiovasc Interv. 2019;12: e007702.
Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7: e011245.
Dinh K, Limmer AM, Paravastu SCV, et al. Mortality after paclitaxel-coated device use in dialysis access: a systematic review and meta-analysis. J Endovasc Ther. 2019;26:600–12.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human or animal participants
This article does not contain any studies with human participants or animals performed by any of the authors. Institutional Review Board approval is not required.
informed consent
For this type of study, informed consent is not required. For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, A.L., Wu, X., Youm, J. et al. Cost-Effectiveness of Drug-Coated Balloon Angioplasty versus Plain Old Balloon Angioplasty for Arteriovenous Fistula Stenosis. Cardiovasc Intervent Radiol 46, 1221–1230 (2023). https://doi.org/10.1007/s00270-023-03403-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-023-03403-3