Abstract
Purpose
To evaluate the preclinical in vivo therapeutic response of Lenvatinib-eluting microspheres (LEN-EM) transcatheter arterial chemoembolization (LEN-TACE) in an hepatocellular carcinoma (HCC) rat model.
Methods
Magnetic resonance imaging (MRI) visible LEN-EM was fabricated with poly(lactide-co-glycolide) and iron oxide nanoparticles by a double-emulsion method. The morphology, LEN loading/release kinetics, and MRI contrast effect of LEN-EM were evaluated. For in vivo study, N1S1 HCC rats were treated with LEN-TACE (LEN: 2.4 mg/kg, n = 5) using LEN-EM, systemic LEN (LEN: 0.4 mg/kg, oral gavage daily for 7 days, n = 5), control (intra-arterial (IA) saline infusion, n = 5), and non-tumor control (n = 3). Tumor size changes were measured for 2 weeks. Histology, comparative LEN plasma concentration, hematologic markers, liver profile, and serum chemistry among the groups were measured.
Results
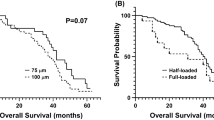
LEN-EM with 33 µm in average size was prepared in an optimized emulsion process. LEN loading efficiency was 58.7%. LEN was continuously released for 500 h. LEN-TACE showed the delivered LEN-EM surrounding tumor tissue in MRI-T2* images. The LEN-TACE group demonstrated a statistically significant larger tumor volume reduction compared to the other groups at 2 weeks post-procedure. Quantification data of Terminal deoxynucleotidyl transferase dUTP nick end labeling positive cells confirmed increased cancer cell death in the LEN-TACE group compared to control groups. Additional histology, hematologic markers, and liver profiles showed minimal side effects of LEN-TACE.
Conclusion
LEN-TACE using IA delivery of LEN-EM demonstrated an effective therapeutic efficacy in an HCC rat animal model.



Similar content being viewed by others
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- IA:
-
Intra-artery
- LEN:
-
Lenvatinib
- LEN-Ems:
-
Lenvatinib-eluting microspheres
- TACE:
-
Transcatheter arterial chemoembolization
- HIF-1a:
-
Hypoxia-inducible factor-1a
- VEGF:
-
Vascular endothelial growth factor
- FDA:
-
Food and Drug Administration
- VEGFR:
-
Vascular endothelial growth factor receptor
- FGFR:
-
Fibroblast growth factor receptor
- IONP:
-
Iron oxide nanoparticles
- PLGA:
-
Poly(lactide-co-glycolide)
- PBS:
-
Phosphate-buffered saline
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- SDMA:
-
Serum symmetric dimethylarginine
References
Kim E, Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med. 2020;52(12):1898–907.
Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103(4):914–21.
Li Z, Hu DY, Chu Q, Wu JH, Gao C, Zhang YQ, Huang YR. Cell apoptosis and regeneration of hepatocellular carcinoma after transarterial chemoembolization. World J Gastroenterol: WJG. 2004;10(13):1876–80.
Han G, Yang J, Shao G, Teng G, Wang M, Yang J, Liu Z, Feng G, Yang R, Lu L, Chao Y, Wang J. Sorafenib in combination with transarterial chemoembolization in Chinese patients with hepatocellular carcinoma: a subgroup interim analysis of the START trial. Future Oncol. 2013;9(3):403–10.
Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14(8):585–7.
Chao Y, Chung YÄ, Han G, Yoon JÄ, Yang J, Wang J, Shao GÄ, Kim BI, Lee TÄ. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136(6):1458–67.
Geschwind JF. Chemoembolization for hepatocellular carcinoma: where does the truth lie? J Vasc Intervent Radiol: JVIR. 2002;13(10):991–4.
Geschwind JF, Ramsey DE, Choti MA, Thuluvath PJ, Huncharek MS. Chemoembolization of hepatocellular carcinoma: results of a metaanalysis. Am J Clin Oncol. 2003;26(4):344–9.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–42.
Chamberlain MN, Gray BN, Heggie JC, Chmiel RL, Bennett RC. Hepatic metastases: a physiological approach to treatment. Br J Surg. 1983;70(10):596–8.
Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K, Shigenobu S, Ishibashi K, Arima T. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97(5):1253–62.
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–71.
Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10(19):2878–82.
Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8(5):292–301.
Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4(6):423–36.
Ma J, Waxman DJ. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther. 2008;7(12):3670–84.
Teoh D, Secord AA. Antiangiogenic agents in combination with chemotherapy for the treatment of epithelial ovarian cancer. Int J Gynecol Cancer. 2012;22(3):348–59.
Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, Tominaga L, Kajiwara A, Kasuya K, Iritani S, Fujiyama S, Hosaka T, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Ikeda K, Arase Y, Hashimoto M, Kozuka T, Kumada H. Lenvatinib-transarterial chemoembolization sequential therapy as an effective treatment at progression during lenvatinib therapy for advanced hepatocellular carcinoma. Liver Cancer. 2020;9(6):756–70.
Ando Y, Kawaoka T, Amioka K, Naruto K, Ogawa Y, Yoshikawa Y, Kikukawa C, Kosaka Y, Uchikawa S, Morio K, Fujino H, Nakahara T, Murakami E, Yamauchi M, Tsuge M, Hiramatsu A, Fukuhara T, Mori N, Takaki S, Tsuji K, Nonaka M, Hyogo H, Aisaka Y, Masaki K, Honda Y, Moriya T, Naeshiro N, Takahashi S, Imamura M, Chayama K, Aikata H. Efficacy and safety of lenvatinib-transcatheter arterial chemoembolization sequential therapy for patients with intermediate-stage hepatocellular carcinoma. Oncology. 2021;99(8):507–17.
Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, Baron A, Park J-W, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng A-L. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73.
Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, Aikata H, Kawaguchi Y, Wada Y, Numata K, Inaba Y, Kuromatsu R, Kobayashi M, Okusaka T, Tamai T, Kitamura C, Saito K, Haruna K, Okita K, Kumada H. REFLECT: a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020;55(1):113–22.
Zhang W, Choi H, Yu B, Kim D-H. Synthesis of iron oxide nanocube patched Janus magnetic nanocarriers for cancer therapeutic applications. Chem Commun. 2020;56(62):8810–3.
Park W, Gordon AC, Cho S, Huang X, Harris KR, Larson AC, Kim D-H. Immunomodulatory magnetic microspheres for augmenting tumor-specific infiltration of natural killer (NK) cells. ACS Appl Mater Interfaces. 2017;9(16):13819–24.
Park W, Cho S, Ji J, Lewandowski RJ, Larson AC, Kim D-H. Development and validation of sorafenib-eluting microspheres to enhance therapeutic efficacy of transcatheter arterial chemoembolization in a rat model of hepatocellular carcinoma. Radiol: Imaging Cancer. 2021;3(1):e200006.
Kim D-H, Li W, Chen J, Zhang Z, Green RM, Huang S, Larson AC. Multimodal imaging of nanocomposite microspheres for transcatheter intra-arterial drug delivery to liver tumors. Sci Rep. 2016;6(1):29653.
Park W, Chen J, Cho S, Park S-J, Larson AC, Na K, Kim D-H. Acidic pH-triggered drug-eluting nanocomposites for magnetic resonance imaging-monitored intra-arterial drug delivery to hepatocellular carcinoma. ACS Appl Mater Interfaces. 2016;8(20):12711–9.
Chen J, White SB, Harris KR, Li W, Yap JW, Kim D-H, Lewandowski RJ, Shea LD, Larson AC. Poly (lactide-co-glycolide) microspheres for MRI-monitored delivery of sorafenib in a rabbit VX2 model. Biomaterials. 2015;61:299–306.
Hiraoka A, Kumada T, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Tada T, Toyoda H, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Imai M, Joko K, Tanaka H, Tamai T, Koizumi Y, Hiasa Y, Michitaka K, Kudo M, Real-life Practice Experts for, H. C. C. S. G., Group, H. C. C. Important clinical factors in sequential therapy including lenvatinib against unresectable hepatocellular carcinoma. Oncology 2019;97(5), 277–85.
Dawkins J, Webster RM. The hepatocellular carcinoma market. Nat Rev Drug Discov. 2019;18(1):13–4.
Llovet JM, Montal R, Villanueva A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J Hepatol. 2019;70(6):1262–77.
Park W, Cho S, Ji J, Lewandowski RJ, Larson AC, Kim DH. Development and validation of sorafenib-eluting microspheres to enhance therapeutic efficacy of transcatheter arterial chemoembolization in a rat model of hepatocellular carcinoma. Radiol Imaging Cancer. 2021;3(1): e200006.
Chen J, Sheu AY, Li W, Zhang Z, Kim DH, Lewandowski RJ, Omary RA, Shea LD, Larson AC. Poly(lactide-co-glycolide) microspheres for MRI-monitored transcatheter delivery of sorafenib to liver tumors. J Control Release. 2014;184:10–7.
Chen J, White SB, Harris KR, Li W, Yap JW, Kim DH, Lewandowski RJ, Shea LD, Larson AC. Poly(lactide-co-glycolide) microspheres for MRI-monitored delivery of sorafenib in a rabbit VX2 model. Biomaterials. 2015;61:299–306.
Ackerman NB. Experimental studies on the circulation dynamics of intrahepatic tumor blood supply. Cancer. 1972;29(2):435–9.
Kim D-H, Chen J, Omary RA, Larson AC. MRI visible drug eluting magnetic microspheres for transcatheter intra-arterial delivery to liver tumors. Theranostics. 2015;5(5):477–88.
Kim DH, Choy T, Huang S, Green RM, Omary RA, Larson AC. Microfluidic fabrication of 6-methoxyethylamino numonafide-eluting magnetic microspheres. Acta Biomater. 2014;10(2):742–50.
Cho S, Min NG, Park W, Kim S-H, Kim D-H. Janus Microcarriers for magnetic field-controlled combination chemotherapy of hepatocellular carcinoma. Adv Func Mater. 2019;29(26):1901384.
Acknowledgements
This work was mainly supported by grants R01CA218659 and R01EB026207 from the National Cancer Institute and National Institute of Biomedical Imaging and Bioengineering. Also, this work was supported by the Center for Translational Imaging and Mouse Histology and Phenotyping Laboratory at Northwestern University. Illustrations were originally created by authors through Biorender.
Funding
This study was funded by grants R01CA218659 and R01EB026207 from the National Cancer Institute and National Institute of Biomedical Imaging and Bioengineering.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for Publication
For this type of study, consent for publication is not required.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed Consent
For this type of study, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pe, J., Choi, B., Choi, H. et al. Preclinical Therapeutic Evaluation of Lenvatinib-Eluting Microspheres for Transcatheter Arterial Chemoembolization of Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 45, 1834–1841 (2022). https://doi.org/10.1007/s00270-022-03242-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03242-8