Abstract
Since its first suggestion as possible option for liver radioembolization treatment, the therapeutic isotope holmium-166 (166Ho) caught the experts’ attention due to its imaging possibilities. Being not only a beta, but also a gamma emitter and a lanthanide, 166Ho can be imaged using single-photon emission computed tomography and magnetic resonance imaging, respectively. Another advantage of 166Ho is the possibility to perform the scout and treatment procedure with the same particle. This prospect paves the way to an individualized treatment procedure, gaining more control over dosimetry-based patient selection and treatment planning. In this review, an overview on 166Ho liver radioembolization will be presented. The current clinical workflow, together with the most relevant clinical findings and the future prospective will be provided.
Similar content being viewed by others
Introduction
Radioembolization, also known as selective internal radiation therapy, is a minimally invasive procedure that combines low-volume embolization and radiation to treat liver cancer. This procedure relies on the principle that hepatic tumors are mainly supplied by hepatic arteries [1]. Thus, radioactive microspheres will predominantly lodge in and around tumorous tissue, sparing healthy liver tissue.
The possibilities to use holmium-166 (166Ho) as a potential isotope for the internal radiation therapy of hepatic tumors was first proposed in 1991 by Mumper et al. [2]. Shortly after, Turner et al. [3] investigated single-photon emission computed tomography (SPECT) dosimetry in pigs, while in 2001 Nijsen et al. [4] performed liver tumor targeting in rats by selective delivery of 166Ho microspheres. Following these promising results in animals’ studies, in 2010 Smits et al. [5] designed the first phase I human trial to evaluate the safety and toxicity profile of 166Ho radioembolization. Since the first publication on 166Ho liver radioembolization, there was a growing interest in this treatment possibility, especially in the last years, as suggested by the increasing publications on this topic.
Holmium-166 Isotope
166Ho microspheres were developed at the Department of Radiology and Nuclear Medicine of the University Medical Center Utrecht and were granted a patent in 2007. In 2015, 166Ho microspheres received CE mark under the commercial name of QuiremSpheres™ (Quirem BV, Deventer, the Netherlands). A test dose of 250 MBq 166Ho microspheres, identical to the ones used for the treatment, received CE mark in 2018 as QuiremScout™ (Quirem BV, Deventer, the Netherlands). A scout dose of 166Ho microspheres can be used to safely evaluate the distribution of intra-arterially injected microspheres prior to treatment and eventually adjust it. In terms of clinical application in liver tumors, 166Ho microspheres are an alternative to the existing yttrium-90 (90Y) devices (either resin or glass).
Physical and Chemical Properties
166Ho microspheres are made of poly-l-lactic acid (PLLA), containing the isotope 166Ho. First in the microspheres production process, holmium-165 (165Ho) is embedded within the matrix structure of PLLA. Then, a part of 165Ho is activated to 166Ho by neutron activation in a nuclear reactor. 166Ho microspheres characteristics are summarized in Table 1.
Imaging Possibilities
The radioactive isotope 166Ho is a high-energy beta-emitting isotope for therapeutic use, but it also emits primary gamma photons that can be used for SPECT. Furthermore, being a lanthanide, it can be imaged by magnetic resonance imaging (MRI) thanks to its paramagnetic properties, enabling the visualization of its distribution in the liver and quantification of the absorbed tumor dose [6, 7].
Single-Photon Emission Compute Tomography
Upon decay, the isotope 166Ho emits several gamma photons, most of which are 81 keV (abundance 6.6%), 1379 keV (0.9%) or 1581 keV (0.2%). Because 166Ho decays to the stable isotope erbium-166 (166Er) with a half-life of 26.8 h, there is a time constraint on the imaging procedure; it should be performed within 6 days after administration. On the other hand, immediately after administration, there is an abundance of gamma photons that significantly increases detector dead time (time duration during which the gamma camera is unable to detect a new scintillation after a previous event). In particular, using an acquisition and reconstruction protocol commonly applied in clinical practice, a 20% count loss due to dead time was observed around 0.7 GBq injected activity [8]. Thus, dependent on the amount of administered activity, it is advised to perform post-treatment 166Ho SPECT/CT between 2 and 5 days after treatment, aiming at an activity < 0.7 GBq at the time of imaging. To properly image 166Ho using SPECT in clinical practice, acquisition parameters are suggested in Table 2.
Magnetic Resonance Imaging
MRI is independent of administered activity; however, because of artifacts, it is limited to tissue without air and metal. MRI has a higher resolution than SPECT/CT.
The presence of Ho (either 166Ho or 165Ho) accelerates the decay of the \({T}_{2}\) vector of tissue. A linear relationship exists between \({T}_{2}^{*}\) times and Ho concentration [9]. This relationship, called relaxivity (\({R}_{2}^{*}\)), depends on the strength of the main magnetic field of the scanner and the Ho content. For a best estimate of \({R}_{2}^{*}\) (hence of the local concentration of Ho), a dedicated fitting incorporating the estimated initial amplitude of the free induction decay curve (S0-fitting) instead of conventional multigradient echo fitting is suggested [10]. In a retrospective analysis including 14 patients [6], a good correlation was found between the whole liver mean absorbed radiation dose as assessed by MRI and SPECT (correlation coefficient = 0.93; P < 0.001), with MRI recovering 89 ± 19% of the delivered activity. A downside of paramagnetic effects of Ho is that susceptibility artifacts obscure gadolinium enhancement on follow-up MRIs, which makes it harder to assess whether treated tumors (or parts) are still enhancing and thus viable. Additionally, the increase in microsphere size, weight percentage or activated fraction of Ho microspheres and field strength can influence sensitivity and detectability of MRI. The impact of these factors on 166Ho-MRI imaging was investigated in a dedicated work [11].
Clinical Workflow
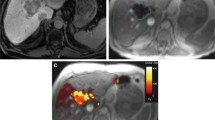
The clinical workflow for 166Ho radioembolization follows similar steps as for radioembolization with other devices. These are summarized in Fig. 1, together with an exemplary clinical case.
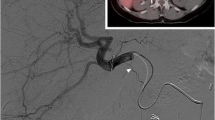
On the left, the steps of the clinical workflow for 166Ho liver radioembolization are depicted. On the right, images referring to an exemplary clinical case are reported. A 73 years old female patient diagnosed with hepatocellular carcinoma was referred for 166Ho radioembolization. Among others, she presented a lesion in segment 6 with a maximum diameter of 71 mm, as it is possible to see from the baseline MRI reported in panel A. During the workup angiography (panel B), coil embolization of the segment 4 artery was performed to obtain intrahepatic redistribution. Consequently, activity initially planned for segment 4 was added to the activity injected in the right hepatic artery, for a total of 122 MBq. In the SPECT/CT acquired after the scout procedure and displayed in panel C, it is possible to see a clear 166Ho uptake in the segment 6, where the tumor lesion was located. No extrahepatic deposition was reported, confirming a successful scout procedure. After having planned the treatment aiming at 60 Gy average absorbed dose to the whole liver (panel D), 4116 166Ho MBq was injected into the right hepatic artery (panel E). 3 days after the treatment, a SPECT/CT was acquired to visually confirm the good targeting of tumor in segment 6 (panel F). Post-treatment dosimetry revealed a good targeting of the tumor, which received a mean dose of 137 Gy, and a safe uptake by the healthy liver, which had a mean absorbed dose of 36 Gy. The MRI acquired 3 months after the treatment (panel G) showed a decrease in lesion size of segment 6 from 71 to 42 mm and complete disappearance of contrast enhancement (complete response according to mRECIST)
Patient Eligibility Assessment
Indications and contraindications for radioembolization with 166Ho-microspheres are the same as for radioembolization with 90Y-microspheres [12] and are summarized in Table 3.
Workup
During a preparatory angiography, the hepatic vasculature is mapped and injection positions are determined. The use of cone-beam CT is of great additional value in this process and helps to determine if there is extrahepatic contrast enhancement. A small batch of 166Ho microspheres with limited activity (200–250 MBq; 60 mg; approximately 3 million 166Ho microspheres) can be used as a scout dose. The safety of 166Ho scout dose in a clinical setting was demonstrated by Braat et al. [13] in a retrospective study including 82 patients. They did not report any relevant clinical toxicity nor adverse events related to an extrahepatic deposition, which occurred in six patients, after a median follow-up of 4 months.
Comparison Between 99mTc-MAA and 166Ho Scout
Traditionally, radioembolization with 90Y requires the use of technetium-99m macroaggregated albumin (99mTc-MAA) as surrogate compound to perform the radioembolization scout procedure. However, 99mTc-MAA differs from the particle used for treatment (either 90Y or 166Ho microspheres) with respect to shape, size, density and number of injected particles, resulting in a different biodistribution. 166Ho radioembolization offers the possibility to use 166Ho microspheres for both scout and treatment procedure, reducing the variables among these and theoretically reducing the discrepancy between the planning and the procedure. 166Ho scout was shown to have a superior predictive value for intrahepatic distribution in comparison with the commonly used 99mTc-MAA [14]. From the analysis of 71 lesions that received two separate scout dose procedures (99mTc-MAA and 166Ho scout), the qualitative analysis showed that 166Ho scout was superior to 99mTc-MAA with a median score of 4 versus 2.5 (P < 0.001). The quantitative analysis showed significantly narrower 95%-limits of agreement for 166Ho scout in comparison with 99mTc-MAA when evaluating lesion absorbed dose (− 90.3 and 105.3 Gy vs. − 164.1 and 197.0 Gy, respectively).
Post-Scout SPECT
The amount of activity injected during the workup procedure is enough for accurate SPECT/CT quantification, but limited enough not to cause tissue damage in case of shunting to the gastrointestinal organs or the lungs [13]. SPECT images are assessed for the presence of extrahepatic depositions in gastrointestinal organs and for lung shunting, and allow for a re-evaluation of the injection positions. Because the same particles are used, lung shunting can be estimated more accurately, as it was demonstrated by Elschot et al. [15] in 14 patients. Using post-treatment 166Ho microspheres SPECT/CT imaging as a reference, pretreatment diagnostic 166Ho microspheres SPECT/CT images were significantly better predictors of the actual lung absorbed doses (reference: median 0.02 Gy, range 0.0–0.7 Gy vs. median 0.02 Gy, range 0.0–0.4 Gy). Doses estimated based on 166Ho microspheres planar scintigraphy (median 10.4 Gy, range 4.0–17.3 Gy), 99mTc-MAA SPECT/CT imaging (median 2.5 Gy, range 1.2–12.3 Gy) and 99mTc-MAA planar scintigraphy (median 5.5 Gy, range 2.3–18.2 Gy) all overestimated the actual lung absorbed doses.
Treatment Planning
The anticipated absorbed dose distribution imaged can be assessed using 166Ho scout, and treatment planning can be adapted based on this distribution. The current activity calculation for 166Ho microspheres is based on a method comparable to the Medical Internal Radiation Dose (MIRD) method. The absorbed dose in Gy delivered by 1 GBq in 1 kg tissue is 15.87 Gy for 166Ho, under the assumption of homogenous distribution in the target volume and absorption of all energy within that volume. The formula for the prescribed activity is based on a 60 Gy average absorbed dose to the whole liver:
According to current instructions for use [16], the average absorbed dose to the perfused volume may exceed 60 Gy (allowing for personalized dosimetry), as long as the average absorbed to the whole liver does not exceed 60 Gy.
Treatment Procedure
After a successful scout procedure, patients undergo treatment with the administration of the treatment dose in a subsequent treatment procedure. Same-day treatment with 166Ho radioembolization is feasible, as proved by van Roekel et al. [17] in 105 patients with a median total procedure time of 6 h and 39 min. On the upside this limits complete treatment to one day, on the downside a same-day approach limits possibilities of personalized treatment based on 166Ho scout distribution since activity needs to be ordered ahead of time.
Post-treatment Evaluation
To assess the outcome of the radioembolization procedure, either a SPECT/CT or MRI can be performed. It allows for the quantification of the dose in the compartments of interest, i.e., tumor and heathy liver, and the evaluation of the dose–response effect. For colorectal cancer patients with inoperable, chemorefractory hepatic metastases, a dose–response threshold was found to be 90 Gy, with sensitivity of 100% and specificity of 38% [18]. Dose–response threshold values for patients with hepatocellular carcinoma and patients with liver metastases of neuroendocrine origin are currently under investigation through the analysis of Hepar Primary and Hepar PLuS data, respectively.
A dedicated software package (Q-suite™, Quirem BV, Deventer, The Netherlands) can be used for treatment planning and dose reconstruction for treatment evaluation.
Two exemplary clinical cases, illustrating the clinical workflow, are summarized in Figs. 2 and 3 showing an hepatocellular carcinoma and a metastatic intrahepatic cholangiocarcinoma, respectively.
85 year old male diagnosed with hepatocellular carcinoma (HCC). At presentation, contrast enhanced T1 MRI (A), a solitary hypervascular lesion in segment 5, 6 and 8 with a maximum diameter of 8.1 cm was seen. At tumor board, the patient was considered for first-line SIRT. The 166Ho scout procedure consisted of a single injection of 233 MBq of 166Ho microspheres in the right hepatic artery (B) and subsequent SPECT/CT imaging showed no lung shunt, no extrahepatic deposition of activity elsewhere and visually good tumor targeting. The patient proceeded with 166Ho treatment in the afternoon (on the same day), in which 4.3 GBq of 166Ho microspheres were administered in the right hepatic artery (B). 3 months after treatment, follow-up contrast enhanced T1 MRI (C), showed a good response reducing its size from 8.1 cm to 5.8 cm and complete response according to mRECIST. Post-treatment SPECT/CT (D) 3 days after treatment confirmed the planned high accumulation of particles in the lesion, without extrahepatic deposition of activity (and no lung shunt). At this moment, more than 3 years after treatment, the patient has no signs of recurrent disease on imaging
64 year old male diagnosed with intrahepatic metastatic cholangiocarcinoma (ICC), with distinct lesions in segment 8, 4 and a minor lesion on the edge of segment 3/4B (A and B). At tumor board, he was considered to be eligible for radioembolization treatment with 166Ho microspheres, which he received after an uneventful 166Ho scout procedure. On the day of treatment a superselective injection of 1.6 GBq (radiation segmentectomy) in segment 8 (C) and segmental injection of 0.8 GBq in segment 4 (D) was executed. Post-treatment SPECT/CT showed a good accumulation of particles around the tumor in segment 8 (E) and segment 4 (F). Contrast enhanced CT (G + H) and 18FDG-PET (not shown) acquired 2 months after treatment showed a near complete regression of the segment 8 lesion and partial response of the segment 4 lesion. Recent follow-up treatment (not shown) consisted of additional 166Ho radioembolization of segment 4 and superselective in segment 3. Segment 8 lesion is still in (near) complete remission
Radiation Safety
As for any procedure that involves the use of radioactive material, the radiation exposure for personnel should be reduced as much as possible based on the ALARA (as-low-as-reasonably-achievable) principal. During treatment, measurements indicated that the additional radiation exposure to staff caused by the 166Ho microspheres procedure is negligible compared to the scattered X-rays from the X-ray tube prior and throughout the procedure [19]. Similar to 90Y procedures, precautionary measures, such as the use of a new microcatheter for each injection position and a fluid-absorbing drape should be considered in order to prevent radioactive contamination. Regulation concerning treatment administration and the release of the angiography suite after a 166Ho treatment vary between centers and countries. Unforeseen 166Ho radioactive contaminations may be more easily detected than 90Y microspheres, because of the primary gamma photon emitted by 166Ho. Depending on the amount of administered therapeutic activity, patients can be released after treatment with minimal contact restrictions (2 days), based on reduction of radiation by distance and time and in consensus with the instructions by the Nuclear Regulatory Commission for patients with permanent implants. 48 h after infusion, exposure rate and activity excretion have been assessed. Exposure rate at discharge, assessed in 15 patients, was 26 μSv/h, which, extrapolated to a whole liver dose of 60 Gy, would lead to a total effective dose equivalent < 5 mSv [20]. Renal and intestinal 166Ho activity excretion was found in all four cases under investigation, independent of the activity of the injected microspheres. The highest total excretion fraction was 0.005% of the injected activity with intestinal excretion being lower than renal excretion [21]. Bakker et al. [22], assessing 1-h blood plasma and 24-h urine, found the median percentage of 166Ho compared to the total amount injected to be 0.19% and 0.32%, respectively.
Clinical Studies on 166Ho Radioembolization
From 2009, eight clinical studies using 166Ho microspheres for radioembolization have been carried out. Type of study, patients’ population, study phase and design, and primary objective are summarized in Fig. 4. Six other studies, mainly exploring the additional value of individualized treatment are currently in preparation. The findings regarding the primary end-point of the prospective studies completed within 2021 are summarized in Table 4. The first study in humans, a dose escalation study, identified the maximum tolerated dose for 166Ho radioembolization at 60 Gy, using the current MIRD method [23]. In addition, it was demonstrated that in vivo dosimetry was feasible by both SPECT and MRI imaging [7]. Subsequently, a phase II study investigated 166Ho radioembolization efficacy [24]. A total of 73% of the study population showed complete response, partial response or stable disease at three-month follow-up, with a median overall survival of 14.5 months, confirming safety and showing efficacy. Another phase II study showed that additional 166Ho radioembolization after peptide receptor radionuclide therapy in patients with metastatic liver neuroendocrine neoplasms is safe and efficacious [25]. Specifically, 43% of patient population achieved an objective response in the treated volume, according to the per-protocol analysis. In nine patients suffering from hepatocellular carcinomas, Radosa et al. [26] showed that 166Ho radioembolization is a feasible and safe treatment option with no significant hepatotoxicity. At six-month follow-up, 89% of patients showed either a complete response, partial response or stable disease. A within-patient randomized study aiming at assessing whether the use of an anti-reflux catheter improves tumor targeting for colorectal cancer patients treated with 166Ho radioembolization confirmed efficacy and toxicity findings of previous studies. Laboratory toxicity was reported for 14% of the patients, while clinical toxicity was found in 19%. One patient (5%) died due to radioembolization-induced liver disease. Median overall survival was 7.8 months. At a tumor-level, a significant dose–response relationship was established with mean tumor-absorbed dose in tumors with complete metabolic response 138% higher, on average, than in progressive tumors (222 Gy vs. 103 Gy, respectively). [27]. To conclude, a phase II study assessing toxicity profile of 166Ho in patients with hepatocellular carcinoma reported unacceptable toxicity in 10% of the treated patients, but no cases of radioembolization-induced liver disease [28]. Additionally, target liver lesions with complete or partial response were found to be 54% and 84% at three- and six-month follow-up, respectively. Median overall survival was 14.9 months. An observational retrospective study recently started (RECORD), aims at further describe the general safety and clinical performance of 166Ho microspheres, with specific attention to outcomes per tumor origin.
Future Prospective
Many possibilities offered by 166Ho liver radioembolization are still to be exploited, especially in clinical practice. Here, the three main directions following from current research are summarized.
Individualized Treatment
Personalized medicine is the Holy Grail that health care providers would like to reach in the near future to optimize patients’ treatment. In the frame of 166Ho radioembolization, this means establishing dose thresholds for patient selection and treatment planning. The definition of robust dose–response values, combined with the use of partition modeling, makes 166Ho the desired isotope when it is preferred to perform scout and treatment procedures using the same particle and for quantitative imaging by SPECT or MRI. While retrospective analysis on a dose–response relationship have been recently published [18, 29], prospective studies are currently in preparation. In particular, the recently registered iHEPAR study focuses on assessing the safety of dosimetry-based individualized treatment planning, which has the potential of improved treatment outcomes. However, individualized treatment planning inherently leads to treatment doses that deviate from the currently approved “one-size-fits-all” approach (i.e., 60 Gy average absorbed dose for all patients). Therefore, safety of individualized 166Ho radioembolization will be evaluated first to validate safety and confirm safety thresholds.
Dual Isotope
The possibility to simultaneously use two isotopes to identify healthy liver and tumorous tissue was firstly suggested by Lam et al. [30]. A protocol including 166Ho scout for treatment simulation and technetium-99m (99mTc) stannous phytate (accumulating in the healthy liver) for healthy liver delineation was proposed to allow for automatic healthy liver segmentation (see Fig. 5). This would avoid the definition of tumor and non-tumorous liver segmentation and registration of a separately acquired contrast enhanced CT or MRI, a time-consuming and prone-to-error task, which is currently necessary to apply the partition modeling enabling personalized activity calculation. The feasibility of this protocol was proved by van Rooij et al. [31] using a phantom study and a proof-of-concept clinical case. For a high accuracy in both 166Ho and 99mTc reconstruction, they suggested a 166Ho:99mTc activity ratio of 5:1. In a phantom experiment, this yielded to a reduction of quantitative 166Ho activity recovery by 10% due to the presence of 99mTc. The possibility to use the dual isotope protocol in a clinical setting without hampering the 166Ho dosimetry has been demonstrated on 65 clinical procedures [32]. The impact of different 99mTc activity on 166Ho quantitative reconstructions and the best method to automatically segment the healthy liver are currently under investigation.
Dual isotope workflow. Firstly, 166Ho microspheres are injected (during either the scout or the treatment procedure), lodging primarily in the tumorous tissues. Additionally, 99mTc-stannous phytate is injected on the SPECT table, accumulating in the Kupffer cells representing the healthy liver tissue. Then a conventional SPECT/CT is acquired that simultaneously acquires two isotopes (166Ho and 99mTc), after which the images are reconstructed correcting the reciprocal scatter caused by the concomitant presence of the two isotopes. These reconstructions are intrinsically registered and thus can be used to automatically define treated tumors and healthy liver avoiding segmentation and registration of a separately acquired CT, which is time-consuming and prone to error
166Ho Radioembolization Under MRI Guidance
The advantages of 166Ho being paramagnetic are not limited to the possibilities to perform quantitative analysis regarding 166Ho dosimetry after the treatment. It also enables an MR guided intratumoral 166Ho microspheres injection. With the possibility to perform three-dimensional visualization of the tumor, a controlled intratumoral needle placement and visual monitoring of the resulting distribution, it offers for a promising improvement of intratumoral holmium treatment [33]. However, further investigation and fine-tuning of the technique is required to make this method suitable for clinical use.
Conclusion
Since their introduction as an alternative to 90Y microspheres, 166Ho microspheres showed unique imaging properties. Additionally, using the 166Ho microspheres for both pretreatment and treatment has the benefit of improving the intrahepatic distribution prediction in comparison with current clinical standard. The combination of these features would enable a better patient selection and individualized treatment planning, paving the way to personalized medicine. To this purpose, safety and efficacy dose thresholds should be further investigated, together with the possibility to fully automatize the segmentation and registration processes necessary for adoption of partition modeling for activity calculation.
Abbreviations
- 166Ho:
-
Holmium-166
- 90Y:
-
Yttrium-90
- 99mTc:
-
Technetium-99m
- CE:
-
Conformitè Europëenne
- CT:
-
Computed tomography
- PLLA:
-
Poly-l-lactic acid
- MAA:
-
Macro aggregated albumin
- MIRD:
-
Medical internal radiation dose
- MRI:
-
Magnetic resonance imaging
- SPECT:
-
Single-photon emission computed tomography
References
Riemenschneider T, Ruf C, Kratzsch HC, Ziegler M, Späth G. Arterial, portal or combined arterio-portal regional chemotherapy in experimental liver tumours? J Cancer Res Clin Oncol. 1992;118(8):597–600.
Mumper RJ, Ryo UY, Jay M. Acid microspheres: a potential agent for the internal radiation therapy of hepatic tumors. J Nucl Med. 1991;32(11):2139–43.
Turner JH, et al. 166Ho-Microsphere liver radiotherapy: a preclinical SPECT dosimetry study in the pig. Nucl Med Commun. 1994;15(7):545–53.
Nijsen F, et al. Targeting of liver tumour in rats by selective delivery of holmium-166 loaded microspheres: a biodistribution study. Eur J Nucl Med. 2001;28(6):743–9.
Smits ML, et al. Holmium-166 radioembolization for the treatment of patients with liver metastases: design of the phase I HEPAR trial. J Exp Clin Cancer Res. 2010;29(1):70.
Van De Maat GH, et al. MRI-Based biodistribution assessment of holmium-166 poly(l-lactic acid) microspheres after radioembolisation. Eur Radiol. 2013;23(3):827–35.
Smits MLJ, et al. In vivo dosimetry based on SPECT and MR imaging of 166Ho—microspheres for treatment of liver malignancies. J Nucl Med. 2013;54(12):2093–100.
Stella M, Braat AJAT, Lam MGEH, de Jong HWAM, van Rooij R. Gamma camera characterization at high holmium-166 activity in liver radioembolization. EJNMMI Phys. 2021;8:1.
Nijsen JFW, Seppenwoolde J-H, Havenith T, Bos C, Bakker CJG, van het Schip AD. Liver tumors: MR Imaging of radioactive holmium microspheres—phantom and rabbit study. Radiology. 2004;231(2):491–9.
Van De Maat GH, Seevinck PR, Bos C, Bakker CJG. Quantification of holmium-166 loaded microspheres: estimating high local concentrations using a conventional multiple gradient echo sequence with S 0-fitting. J Magn Reson Imaging. 2012;35(6):1453–61.
Seevinck PR, et al. Factors affecting the sensitivity and detection limits of MRI, CT, and SPECT for multimodal diagnostic and therapeutic agents. Anticancer Agents Med Chem. 2008;7(3):317–34.
Kennedy A, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68(1):13–23.
Braat AJAT, Prince JF, van Rooij R, Bruijnen RCG, van den Bosch MAAJ, Lam MGEH. Safety analysis of holmium-166 microsphere scout dose imaging during radioembolisation work-up: a cohort study. Eur Radiol. 2018;28(3):920–8.
Smits MLJ, et al. The superior predictive value of 166Ho-scout compared with 99mTc-macroaggregated albumin prior to 166Ho-microspheres radioembolization in patients with liver metastases. Eur J Nucl Med Mol Imaging. 2020;47(4):798–806.
Elschot M, et al. 99mTc-MAA overestimates the absorbed dose to the lungs in radioembolization: a quantitative evaluation in patients treated with 166Ho-microspheres. Eur J Nucl Med Mol Imaging. 2014;41(10):1965–75.
Quirem Medical B.V. QuiremSpheres®—instructions for use. 2020. p. 2.
van Roekel C, et al. Evaluation of the safety and feasibility of same-day holmium-166—radioembolization simulation and treatment of hepatic metastases. J Vasc Interv Radiol. 2020;31(10):1593–9.
van Roekel C, et al. Dose-effect relationships of 166Ho radioembolization in colorectal cancer. J Nucl Med. 2021;62(2):272–9.
Reinders MTM, Smits MLJ, van Roekel C, Braat AJAT. Holmium-166 microsphere radioembolization of hepatic malignancies. Semin Nucl Med. 2019;49(3):237–43.
Prince JF, et al. Radiation emission from patients treated with holmium-166 radioembolization. J Vasc Interv Radiol. 2014;25(12):1956-1963.e1.
Drescher R, Kühnel C, Seifert P, Gühne F, Freesmeyer M. Renal and Intestinal excretion of 90 y and 166 Ho after transarterial radioembolization of liver tumors. Am J Roentgenol. 2020;214(5):1158–64.
Bakker RC, et al. Blood and urine analyses after radioembolization of liver malignancies with [166Ho] Ho-acetylacetonate-poly(l-lactic acid) microspheres. Nucl Med Biol. 2019;71:11–8.
Smits MLJ, et al. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol. 2012;13(10):1025–34.
Prince JF, et al. Efficacy of radioembolization with 166 Ho-microspheres in salvage patients with liver metastases: a phase 2 Study. J Nucl Med. 2018;59(4):582–8.
Braat AJAT, et al. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (HEPAR PLuS): a single-centre, single-arm, open-label, phase 2 study. Lancet Oncol. 2020;21(4):561–70.
Radosa CG, et al. Holmium-166 radioembolization in hepatocellular carcinoma: feasibility and safety of a new treatment option in clinical practice. Cardiovasc Interv Radiol. 2019;42(3):405–12.
van Roekel C, et al. Use of an anti-reflux catheter to improve tumor targeting for holmium-166 radioembolization—a prospective, within-patient randomized study. Eur J Nucl Med Mol Imaging. 2021;48(5):1658–68.
Reinders-Hut MTM, et al. Safety and efficacy of holmium-166 radioembolization in hepatocellular carcinoma: the HEPAR Primary study. Cardiovasc Interv Radiol. 2021;44(S1):1–64.
Bastiaannet R, et al. First evidence for a dose-response relationship in patients treated with 166Ho radioembolization: a prospective study. J Nucl Med. 2020;61(4):608–12.
Lam MGEH, et al. Fusion dual-tracer SPECT-based hepatic dosimetry predicts outcome after radioembolization for a wide range of tumour cell types. Eur J Nucl Med Mol Imaging. 2015;42(8):1192–201.
van Rooij R, Braat AJAT, de Jong HWAM, Lam MGEH. Simultaneous 166Ho/99mTc dual-isotope SPECT with monte carlo-based downscatter correction for automatic liver dosimetry in radioembolization. EJNMMI Phys. 2020;7(1):13.
Stella M, Braat A, Lam M, de Jong H, van Rooij R. Quantitative 166Ho-microspheres SPECT derived from a dual-isotope acquisition with 99mTc-colloid is clinically feasible. EJNMMI Phys. 2020;7(1):48.
Reijniers NCB. The spatial biodistribution and dosimetry of holmium loaded microspheres after intratumoral injection. 2014. p. 110
Acknowledgements
The authors thank Eline Ekkelenkamp for the information about clinical studies involving holmium-166.
Funding
Funding for this study was received from NWO (Dutch Research Council), Project Number NWA.ID.17.059.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MS is employed by the UMC Utrecht under a collaborative grant of the Dutch Research Council (NWO) between UMC Utrecht and Quirem Medical BV. RvR and HWAMdJ have acted as a consultant for BTG/Boston Scientific. AJATB has acted as consultant for BTG/Boston Scientific and Terumo. MGEHL has acted as a consultant for BTG/Boston Scientific and Terumo, and receives research support from BTG/Boston Scientific and Quirem Medical BV. The Department of Radiology and Nuclear Medicine of the UMC Utrecht receives royalties from Quirem Medical BV. No other potential conflicts of interest relevant to this article exist.
Informed Consent
Informed consent was obtained from all individual participants included in the studies mentioned in this review.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in this review.
Ethical Approval
All procedures performed in studies mentioned in this review were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of review, additional informed consent is not required. The clinical studies mentioned in this review are registered with Clinicaltrials.gov as: Hepar I-NCT01031784-15th December 2009; Hepar II-NCT01612325-5th June 2012; Hepar PLuS-NCT02067988-20th February 2014; SIM-NCT02208804-5th August 2014; Hepar Primary-NCT03379844-20th December 2017; HORA EST-NCT03437382-19th February 2018; Emeritus-NCT04269499-13th February 2020; RECORD-NCT05111795-8th November 2021; iHepar-NCT05114148-9th November 2021.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stella, M., Braat, A.J.A.T., van Rooij, R. et al. Holmium-166 Radioembolization: Current Status and Future Prospective. Cardiovasc Intervent Radiol 45, 1634–1645 (2022). https://doi.org/10.1007/s00270-022-03187-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03187-y