Abstract
Purpose
To compare the recanalization of the uterine arteries and uterine necrosis after uterine artery embolization (UAE) using either soluble gelatin sponge particles (SGS), which dissolve in saline, or tris-acryl gelatin microspheres (MS), which are permanent embolic materials, in swine.
Methods
Fourteen uteri in seven swine were divided into two groups for embolization with either 500–1000 µm SGS (SGS group) or 500–700 µm MS (MS group) (seven uteri per group). The uterine arteries were embolized using SGS or MS, and angiography was performed to evaluate recanalization of the uterine arteries immediately, 1, 2, 3, 4, 5, and 6 h, and 3 days after embolization. On day 3, the uteri were removed to determine the macroscopic necrosis rate and for histopathologic examination.
Results
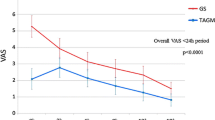
In the SGS group, four uterine arteries were completely recanalized, two were partially recanalized, and one was still occluded 5 h after embolization. In contrast, all seven uterine arteries in the MS group were still occluded 6 h after embolization. The complete recanalization rate at 3 days was significantly greater in the SGS group than in the MS group (100.0% vs. 14.3%, respectively; P = .0047). The mean uterine necrosis rate was not significantly different between the SGS and MS groups (15.0 ± 15.7% vs. 26.8 ± 13.3%, respectively; P = .096). The mean smallest arterial diameter containing embolic materials was 48.2 ± 22.0 μm (range 21–109 μm) for SGS and 446.7 ± 107.0 μm (range 352–742 μm) for MS (P < .0001).
Conclusion
The uterine arteries recanalized earlier in the SGS group than in the MS group and the uterine necrosis rates were similar in both groups. SGS have the potential for a more distal penetration in comparison with MS.






Similar content being viewed by others
References
Silberzweig JE, Powell DK, Matsumoto AH, Spies JB. Management of uterine fibroids: a focus on uterine-sparing interventional techniques. Radiology. 2016;280:675–92.
Keung JJ, Spies JB, Caridi TM. Uterine artery embolization: A review of current concepts. Best Pract Res Clin Obstet Gynaecol. 2018;46:66–73.
de Bruijn AM, Ankum WM, Reekers JA, et al. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 10-year outcomes from the randomized EMMY trial. Am J Obstet Gynecol. 2016;215:745.
Ananthakrishnan G, Murray L, Ritchie M, et al. Randomized comparison of uterine artery embolization (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): subanalysis of 5-year MRI findings. Cardiovasc Intervent Radiol. 2013;36:676–81.
Manyonda IT, Bratby M, Horst JS, et al. Uterine artery embolization versus myomectomy: impact on quality of life–results of the FUME (Fibroids of the Uterus: Myomectomy versus Embolization) Trial. Cardiovasc Intervent Radiol. 2012;35:530–6.
Pron G, Mocarski E, Bennett J, et al. Tolerance, hospital stay, and recovery after uterine artery embolization for fibroids: the Ontario Uterine Fibroid Embolization Trial. J Vasc Interv Radiol. 2003;14:1243–50.
Spencer EB, Stratil P, Mizones H. Clinical and periprocedural pain management for uterine artery embolization. Semin Intervent Radiol. 2013;30:354–63.
McLucas B, Voorhees WD 3rd, Elliott S. Fertility after uterine artery embolization: a review. Minim Invasive Ther Allied Technol. 2016;25:1–7.
Mohan PP, Hamblin MH, Vogelzang RL. Uterine artery embolization and its effect on fertility. J Vasc Interv Radiol. 2013;24:925–30.
Berkane N, Moutafoff-Borie C. Impact of previous uterine artery embolization on fertility. Curr Opin Obstet Gynecol. 2010;22:242–7.
Ghanaati H, Sanaati M, Shakiba M, et al. Pregnancy and its outcomes in patients after uterine fibroid embolization: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2020;43:1122–33.
Das R, Champaneria R, Daniels JP, Belli AM. Comparison of embolic agents used in uterine artery embolisation: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2014;37:1179–90.
Blaine G. Absorbable gelatin sponge in experimental surgery. Lancet. 1951;258:427–9.
Yadavali R, Ananthakrishnan G, Sim M, et al. Randomised trial of two embolic agents for uterine artery embolisation for fibroids: Gelfoam versus Embospheres (RAGE trial). CVIR Endovasc. 2019;2:4.
Takasaka I, Kawai N, Sato M, et al. A new soluble gelatin sponge for transcatheter hepatic arterial embolization. Cardiovasc Intervent Radiol. 2010;33:1198–204.
Kanayama Y, Aoki C, Sakai Y. Development of low endotoxin gelatin for regenerative medicine. Biol Pharm Bull. 2007;30:237–41.
Sonomura T, Kawai N, Ikoma A, et al. Uterine damage in swine following uterine artery embolization: comparison among gelatin sponge particles and two concentrations of N-butyl cyanoacrylate. Jpn J Radiol. 2013;31:685–92.
Sone M, Osuga K, Shimazu K, et al. Porous gelatin particles for uterine artery embolization: an experimental study of intra-arterial distribution, uterine necrosis, and inflammation in a porcine model. Cardiovasc Intervent Radiol. 2010;33:1001–8.
Sanda H, Kawai N, Sato M, et al. Synthesis of 24-h soluble gelatin sponge particles and their effect on liver necrosis following hepatic artery embolization: results of in vitro and in vivo studies. Hepatol Res. 2016;46:335–42.
Sato M, Yamada R. Experimental and clinical studies on the hepatic artery embolization for treatment of hepatoma. Nippon Acta Radiologica. 1983;43:977–1005.
Kawai N, Sato M, Minamiguchi H, et al. Clinical evaluation of transcatheter arterial chemoembolization with 2-day-soluble gelatin sponge particles for hepatocellular carcinoma-comparison with insoluble gelatin sponge particles. J Vasc Interv Radiol. 2013;24:1383–90.
Weichert W, Denkert C, Gauruder-Burmester A, et al. Uterine arterial embolization with tris-acryl gelatin microspheres: a histopathologic evaluation. Am J Surg Pathol. 2005;29:955–61.
Sonomura T, Yamada R, Kishi K, et al. Dependency of tissue necrosis on gelatin sponge particle size after canine hepatic artery embolization. Cardiovasc Intervent Radiol. 1997;20:50–3.
Laurent A, Wassef M, Namur J, et al. Recanalization and particle exclusion after embolization of uterine arteries in sheep: a long-term study. Fertil Steril. 2009;91:884–92.
Katsumori T, Miura H, Arima H, et al. Tris-acryl gelatin microspheres versus gelatin sponge particles in uterine artery embolization for leiomyoma. Acta Radiol. 2017;58:834–41.
Ruuskanen A, Sipola P, Hippeläinen M, et al. Pain after uterine fibroid embolisation is associated with the severity of myometrial ischaemia on magnetic resonance imaging. Eur Radiol. 2009;19:2977–85.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sato, H., Sonomura, T., Onishi, S. et al. Comparison of Uterine Necrosis After Uterine Artery Embolization with Soluble Gelatin Sponge Particles or Tris-acryl Gelatin Microspheres in Swine. Cardiovasc Intervent Radiol 44, 1780–1789 (2021). https://doi.org/10.1007/s00270-021-02905-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-021-02905-2