Abstract
Purpose
Although image-guided biopsies of bone and soft tissue lesions have a low complication rate, there is limited data evaluating use of preprocedural laboratory tests. To address this issue, patients were not required to stop non-steroidal antiinflammatory drugs (NSAIDs) and aspirin or to obtain preprocedural laboratory tests [complete blood count (CBC) and international normalized ratio (INR)], except in special circumstances. The bleeding complication rate, rate of same day biopsies, and the time from when the biopsy was ordered to when it was performed were obtained.
Materials and Methods
A total of 332 patients who underwent bone or soft tissue biopsies performed at our institution between 9/1/2017 and 1/9/2019 were prospectively analyzed. These data were compared to a retrospective biopsy cohort of 323 patients between 7/1/2015 and 7/1/2017. Data collected included method of image guidance and bleeding complication rate. The number of days from ordering to performing a biopsy and number of same day biopsies were recorded.
Results
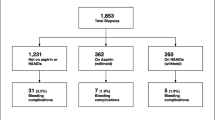
There were no bleeding complications in either cohort (OR 1.00, P = 1). The mean time from ordering to performing a bone biopsy was significantly decreased in the prospective group (6.6 days) compared to the retrospective group (8.1 days) (P = 0.012). There were more same day biopsies in the prospective cohort (11.4% vs. 3.4%) (P < 0.001).
Conclusions
Preprocedural CBC and INR for bone and soft tissue biopsies can be safely eliminated in most patients. Biopsies performed while patients are taking NSAIDs/aspirin can safely be performed. Adopting revised preprocedural laboratory criteria can result in decreased time to completion of biopsies.

Similar content being viewed by others
References
Weidner S, Kellner W, Kellner H. Interventional radiology and the musculoskeletal system. Best Pract Res Clin Rheumatol. 2004;18(6):945–56.
Rosenthal D. The future of MSK interventions. Skeletal Radiol. 2011;40(9):1133–6. https://doi.org/10.1007/s00256-011-1225-0.
Kaur I, Handa U, Kundu R, Garg SK, Mohan H. Role of fine-needle aspiration cytology and core needle biopsy in diagnosing musculoskeletal neoplasms. J Cytol. 2016;33(1):7–12. https://doi.org/10.4103/0970-9371.175478.
Tsukushi S, Nishida Y, Yamada Y, Yoshida M, Ishiguro N. CT guided needle biopsy for musculoskeletal lesions. Arch Orthop Trauma Surg. 2010;130(5):699–703.
Rimondi E, Rossi G, Bartalena T, Ciminari R, Alberghini M, Ruggieri P, et al. Percutaneous CT-guided biopsy of the musculoskeletal system: results of 2027 cases. Eur J Radiol. 2011;77(1):34–42.
Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Percutaneous CT-guided interventional procedures in musculoskeletal system (our experience). Eur J Radiol. 2004;50(3):273–7.
Wang DT, Dubois M, Tutton SM. Complications in musculoskeletal intervention: important considerations. Semin Intervent Radiol. 2015;32(2):163–73. https://doi.org/10.1055/s-0035-1549447.
Huang AJ, Halpern EF, Rosenthal DI. Incidence of delayed complications following percutaneous CT-guided biopsy of bone and soft tissue lesions of the spine and extremities: a 2-year prospective study and analysis of risk factors. Skelet Radiol. 2013;42(1):61–8.
Committee on Standards and Practice Parameters, Apfelbaum JL, Connis RT, Nickinovich DG, American Society of Anesthesiologists Task Force on Preanesthesia Evaluation, Pasternak LR, Arens JF, Caplan RA, Connis RT, Fleisher LA, Flowerdew R, Gold BS, Mayhew JF, Nickinovich DG, Rice LJ, Roizen MF, Twersky RS. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists task force on Preanesthesia evaluation. Anesthesiology. 2012;116(3):522–38.
Rohrer MJ, Michelotti MC, Nahrwold DL. A prospective evaluation of the efficacy of preoperative coagulation testing. Ann Surg. 1988;208(5):554–7.
Foremny GB, Pretell-Mazzini J, Jose J, Subhawong TK. Risk of bleeding associated with interventional musculoskeletal radiology procedures: a comprehensive review of the literature. Skelet Radiol. 2015;44(5):619–27.
Chee YL, Greaves M. Role of coagulation testing in predicting bleeding risk. Hematol J. 2003;4(6):373–8.
Ashkar LK, Hafiz RM. Costly coagulation profile tests prior to performing breast biopsies. Do we really need it? Saudi Med J. 2016;37(6):638–40.
Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG, Saad WA, Standards of Practice Committee, with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727–36.
Rodeghiero F, Castaman G, Dini E. Epidemiological investigation of the prevalence of von Willebrand’s disease. Blood. 1987;69(2):454–9.
Franchini M, Di Perna C, Santoro C, Castaman G, Siboni SM, Zanon E, Linari S, Gresele P, Pasca S, Coppola A, Santoro R, Napolitano M, Ranalli P, Tagliaferri A, Italian Association of Haemophilia Centres. Cancers in patients with von Willebrand disease: a survey from the Italian Association of Haemophilia Centres. Semin Thromb Hemost. 2016;42(1):36–41.
Trieu J, Schlicht SM, Choong PF. Diagnosing musculoskeletal tumours: how accurate is CT-guided core needle biopsy? Eur J Surg Oncol. 2016;42(7):1049–56.
Shif Y, Kung JW, McMahon CJ, Mhuircheartaigh JN, Lin YC, Anderson ME, Wu JS. Safety of omitting routine bleeding tests prior to image-guided musculoskeletal core needle biopsy. Skeletal Radiol. 2018;47(2):215–21.
Liu B, Limback J, Kendall M, Valente M, Armaly J, Grekoski V, Pinizzotto A, Burt J, Ward TJ. Safety of CT-guided bone marrow biopsy in thrombocytopenic patients: a retrospective review. J Vasc Interv Radiol. 2017;28(12):1727–31.
Cardella JF, Bakal CW, Bertino RE, Burke DR, Drooz A, Haskal Z, Lewis CA, Malloy PC, Meranze SG, Oglevie SB, Sacks D, Towbin RB, Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for image-guided percutaneous biopsy in adults. J Vasc Interv Radiol. 2003;14(2):S227–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author S.T. reports royalties for Teleflex and Cook and is a consultant for Adrenas, MedComp, BD Bard, Lutonix, WL Gore, Cook, Teleflex.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehta, S.D., Weber, K., Fleisher, L. et al. Assessing the Need for Preprocedural Laboratory Tests and Stopping Non-steroidal Anti-inflammatory Drugs/Aspirin in Patients Undergoing Percutaneous Bone and Soft Tissue Biopsies. Cardiovasc Intervent Radiol 42, 1588–1596 (2019). https://doi.org/10.1007/s00270-019-02274-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02274-x