Abstract
Purpose
This study was designed to summarize the evidence on clinical outcomes and complications of prostatic arterial embolization (PAE) in patients with benign prostatic hyperplasia (BPH).
Methods
We searched Medline and Embase for PAE trials of patients with BPH upto November 2013. Two reviewers independently checked the inclusion and exclusion criteria and performed data extraction of study characteristics, quantitative and qualitative outcomes, and complications.
Results
The search yielded 562 studies, of which 9 articles with 706 patients were included. In these 9 articles, there was a possible overlap of data and the quality of 8 studies was assessed as poor. All patients had moderate-to-severe, lower urinary tract symptoms (LUTS). The mean age ranged from 63.4–74.1 years. After embolization, a decrease of the prostate volume (PV) and post void residual (PVR) was seen mainly in the first month with a further decrease up to 12 months, increasing afterwards. The prostate specific antigen (PSA) decreased up to 3 months after PAE, increasing afterwards. The peak urinary flow (Qmax) increased mainly the first month and decreased after 30 months. The international prostate symptom score (IPSS) and quality of life-related symptoms (QOL) improved mainly during the first month, with a further improvement up to 30 months. No deterioration of the international index of erectile function (IIEF) was seen after PAE. The PAE procedure seems safe.
Conclusions
Although the number of studies was small, qualitatively poor, and with overlap of patients, the initial clinical outcomes as reported up to 12 months seem positive and the procedure seems safe.
Similar content being viewed by others
Introduction
Benign prostatic hyperplasia (BPH) is common in middle-aged and elderly men [1]. The enlarged gland is an important cause of lower urinary tract symptoms (LUTS), such as a weak urinary stream, higher urinary frequency, intermittent voiding, nocturia, and urinary urgency [2, 3]. The prevalence and severity of LUTS in aging men can be progressive. Approximately 25 % of men in their 50s, 33 % of men in their 60s, and circa 50 % of men at 80 years of age suffer from moderate to severe LUTS [1]. LUTS caused by BPH can have a significant impact on the quality of life (QOL), and when severe, BPH can even lead to acute urinary retention. The severity of LUTS and effect on QOL are important considerations for deciding when treatment is indicated [3–5]. Treatment options include watchful waiting, medical treatment, minimally invasive, or surgical therapies [6]. Medical therapy is usually the first-line treatment option for patients with mild-to-moderate LUTS [7, 8]. In patients with moderate-to-severe LUTS, transurethral resection of the prostate (TURP) is still the “gold standard” surgical treatment for BPH to improve symptoms and decrease progression. However, it is associated with substantial morbidity, such as bleeding, irritative voiding symptoms postoperative, long-term ejaculatory dysfunction, and bladder neck contractures [9].
Prostatic arterial embolization (PAE) gained special attention in the past years as a potential minimally invasive technique for patients with moderate-to-severe LUTS due to BPH. Previous animal studies have shown that PAE can induce prostatic volume reduction and is safe, with no procedure-related sexual dysfunction [10, 11]. In this review, we systematically summarized all evidence on PAE in humans to assess the quantitative clinical outcomes [prostate volume (PV), prostate-specific antigen (PSA), peak urinary flow (Qmax), post void residual (PVR)], qualitative clinical outcomes [International Prostate Symptom Score (IPSS), QOL, and International Index of Erectile Function (IIEF)], and complications related to the procedure.
Materials and Methods
This review was conducted according to the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines [12]. The review protocol was not published or registered in advance.
Search Strategy
Search was performed in Medline and Embase until the end of November 2013. The Medline search included: (Embolisation [MeSH Terms/Title/Abstract] OR Embolization [MeSH Terms/Title/Abstract]) AND (Prostate [Title/Abstract] OR Prostatic [Title/Abstract] OR Prostatic diseases [MeSH Terms] OR Prostate [MeSH Terms]).
The Embase search included: (Artificial Embolism [MeSH Terms] OR Embolization [All field] OR Embolisation [All field])) AND (Prostate [MeSH Terms] OR Prostate disease [MeSH Terms] OR Prostate [All field] OR Prostatic [All field]).
Study Selection
Two independent reviewers (S.M.S. and A.E.S.) selected all potentially relevant studies. Based on titles and abstract, studies were excluded if they were duplicates, reviews, comments, letters, case reports (<5 patients), studies not concerning prostatic hyperplasia, animal studies, conference abstracts, and nonhuman studies. All other studies were considered as potentially relevant and full texts were retrieved. Inclusion and exclusion criteria were checked by two independent reviewers (S.M.S. and A.E.S.), and a third reviewer was consulted (S.B.) in case of disagreement. Studies were included if they contained information about more than five cases of PAE in patients with BPH and if one or more of the following clinical outcomes were evaluated: PV, PSA, Qmax, PVR, IPSS, QOL, and IIEF.
Data Extraction
Data extraction was performed in a standardized manner by using a data extraction form. Two authors (S.M.S. and A.E.S.) extracted the data independently from the included studies. A third reviewer performed the consensus (S.B.).
Study Design Characteristics
The following data on study design characteristics were extracted: (1) Study type (cohort, RCT or other); (2) Data collection (prospective, retrospective, or other); (3) Study design (multicenter/single center); (4) Institutions involved (academic, non-academic); (5) Departments involved (radiology, urology); (6) Period of recruitment; (7) METC approval; and (8) Funding and potential role of funders in study.
Patient Characteristics
The following data on patient characteristics were extracted: (1) Patient population inclusion (consecutive or nonconsecutive); (2) Inclusion and exclusion criteria; (3) Number of patients included; (4) Number of patients analysed; (5) Age of patients; (6) Medication at baseline. Based on all items mentioned above, we defined whether the spectrum of patients was representative for the patients who would receive the embolization in practice.
Embolization Procedure
The following data on the embolization procedures were assessed: (1) Performing physician; (2) Unilateral or bilateral; (3) Embolization material; (4) Procedure time total; (5) Procedure time fluoroscopy, (6) Previous treatment other than medication; (7) Drop outs reported. Based on items 1–5, we defined whether the procedure was described in sufficient detail to permit its replication.
Risk of Bias (Quality Assessment)
For the risk of bias (quality assessment), several items of the study design characteristics, patient population, and embolization procedure were used based on the QUADAS2 tool [13]: study design characteristics: (1) study type (cohort, RCT or other); (2) data collection (prospective, retrospective, or other); (3) study design (multicenter/single-center); (4) METC approval (yes, no, or unclear); and (5) funding and potential role of funders in study (yes, no, or unclear).
Patient characteristics: (1) patient population inclusion (consecutive or nonconsecutive); (2) inclusion and exclusion criteria; (3) whether the spectrum of patients was representative for the patients who would receive the embolization in practice (yes or no).
Embolization procedure: (1) dropouts in study (yes, no, or unclear); and (2) whether the procedure was described in sufficient detail to permit its replication (yes or no).
Sample size: we checked whether sample size calculation was performed (yes or no).
Quality was judged based as follows: study type (0 = cohort or other vs. 1 = RCT), data collection (0 = retrospective or other vs. 1 = prospective), design (0 = single-center vs. 1 = multicenter), METC approval (0 = no or unclear vs. 1 = yes), funding or conflict of interest (0 = yes or unclear vs. 1 = no), inclusion/exclusion criteria defined (0 = no vs. 1 = yes), patients spectrum generalizable (0 = no vs. 1 = yes), dropouts in study (0 = yes or unclear vs. 1 = no), procedure description sufficient (0 = no vs. 1 = yes) and sample size calculation (0 = no vs. 1 = yes). Finally, all points were summed to reach a quality assessment. More than eight points was considered good quality.
Baseline and Follow-Up and Data Extraction on Outcomes
Baseline and follow-up (time, number of patients, dropout description) were recorded, and the following data on outcomes were extracted per given follow-up: (1) Quantitative clinical outcomes (PV, PSA, Qmax, PVR, others): (2) Qualitative clinical outcomes (IPSS, QOL, IIEF, Others); (3) Complications related to the procedure; (4) Other outcomes related to the procedure (technical success, clinical failures, hospitalization, others). All outcomes were continuous data.
The baseline data were presented as means and standard deviation, because these were always reported in the published studies. For the follow-up, we also aimed to present the means and standard deviations; however, these were not always reported.
Data-Analysis
For the comparison of follow-up with the baseline, we present means. If means were not presented, we calculated means first by using the available mean changes (decrease or increase) or second by using the available % change (decrease or increase). Because standard deviation was not available in all datasets, we were not able to pool the results for a meta-analysis approach. Therefore, we calculated pooled weighted mean, taking into account the number of patients and the mean per study to present an overview of the results at baseline and follow-up.
Results
Search Strategy and Study Selection
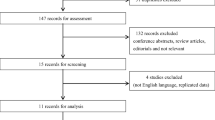
The search yielded 562 studies: 170 in Medline and 392 in Embase. After removing studies not concerning BPH or studies evaluating animals (415), conference papers/letters/comments (51), duplicates (41), reviews/case reports (44), and one article of which the full article was not found, ten studies were selected for full-text review, checking on inclusion and exclusion criteria (Supplement 1). After checking the inclusion and exclusion criteria, one study was excluded based on Chinese language. All data were extracted from the nine studies eligible for the systematic review (Supplement 1) [14–22]. These nine studies were all published by three research groups, with probably an overlap of patients. However, there was no complete duplication of patients (based on inclusion/exclusion criteria, inclusion period, patients’ characteristics), and each study showed differences in outcomes; therefore, all studies were included in this review.
Study Design Characteristics
All studies were performed between June 2008 and March 2013. Of the nine studies, eight were cohort studies [14–17, 19–22]. Only one study, comparing two different sizes of PVA particles, was a randomized, controlled trial [18]. All studies were initiated by a department of radiology, and the majority was supported by a department of urology. There was a variation in prospective and retrospective data collection. In Table 1, all study design characteristics are described.
Funding
No conflict of interest was stated in four studies [15, 16, 21, 22]. Potential conflict of interest was unclear in four studies [14, 17, 18, 20]. In one study, the authors stated a potential conflict of interest (Cook Medical; speaker/honoraria; consultant/advisory board) [19].
Patient Population Characteristics
In total, 706 patients were included. The mean age ranged from 63.4–74.1 years (mean of means is 68.1 years). As stated before, there is probably an overlap in patients. All included patients were diagnosed with BPH and moderate-to-severe LUTS. Malignancy was an exclusion criterion in all studies. Table 2 provides detailed information on patient population.
Embolization Procedure Description
In two studies [18, 22], the authors explicitly stated that the embolization procedure was performed by an interventional radiologist. However, we presume that interventional radiologists also performed the procedure in the other studies. The embolization procedure was performed using 90–180 or 180–300 µm nonspherical polyvinyl alcohol (PVA) particles [14–19], 300–500 µm microspheres [20, 21], or 100–400 µm spherical embolic agents [22]. Mean total procedure time varied from 70.4 to 96.3 min (mean of the means 80.1 min), and the mean fluoroscopy time varied from 18 to 85.9 min (mean of the means 36.5 min). Most studies had the intention to perform the embolization bilaterally; however, in some cases only unilateral embolization was performed due to atherosclerosis. One cohort study compared bilateral versus unilateral embolization [19]. In total, 564 patients underwent a bilateral embolization, 91 a unilateral embolization, and for 22 patients it was unclear if one or both sides were treated [20, 21]. In 13 patients, embolization could not be performed due to tortuosity of vessels. In Table 3, an overview of the embolization procedures are described.
Risk of Bias (Quality Assessment)
Only one study fulfilled our criteria of quality assessment (16). All other studies, including the RCT (18), were rated below 8 points, mainly due to the type of study (mostly cohort, n = 8), unclear inclusion of patients (n = 6), unclear or conflict of interest (n = 5), dropouts (n = 8), and missing sample size calculation (n = 9). Details are given in Table 4.
Follow-up and Data Presentation
Follow-up Intentional follow-up varied between 6 and 24 months. All patients had at least 1 month of follow-up. Detailed data of the number of patients at various times of follow-up is presented in the tables with outcomes.
Quantitative clinical data All data for PV, PSA, Qmax, PVR are presented in Tables 5, 6, 7, 8.
PV The pooled weighted mean PV at baseline was 83.6 ml (range of the means 56.7–104.9). In the first month after embolization, PV decreased to a pooled weighted mean of 66.4 ml (range of the means 44.4–68.7). This decrease persisted up to 12 months after treatment. At further follow-up, PV showed an increase to a pooled weighted mean of 83.7 ml (range of the means 75.9–90.9) at 24 months in 27 patients and 72.0 ml at 30 months in 4 patients. There was no significant effect on PV in patients who underwent unilateral versus bilateral embolization, nor in patients who underwent embolization with 90–180 or 180–300 µm PVA particles [18, 19].
PSA The pooled weighted mean PSA at baseline was 6.28 ng/ml (range of the means 5.6–10.1). The first 3 months after treatment, PSA decreased to a pooled weighted mean of 3.98 ng/ml (range of the means 3.7–6.49). After 6 months follow-up, PSA started to increase to a pooled weighted mean of 5.96 ng/ml (range of the means 5.08–6.24) at 24 months and 7.41 ng/ml at 30 months. A significant greater reduction of PSA was seen in PAE with 90–180 µm PVA particles compared with PAE with 180–300 µm PVA particles (P = 0.001) [18]. Unilateral versus bilateral treatment showed no significant differences [19].
Qmax The pooled weighted mean Qmax at baseline was 8.69 ml/s (range of the means 4.2–9.94). The Qmax increased mainly in the first month after PAE to a pooled weighted mean of 12.00 ml/s (range of the means 11.6–13). This increase persisted up to 18 months, whereas at 30 months the Qmax decreased to 10.80 ml/s in two patients. There was no significant effect on Qmax in patients who underwent unilateral versus bilateral embolization, nor in patients who underwent embolization with 90–180 or 180–300 µm PVA particles [18, 19].
PVR The pooled weighted mean PVR at baseline was 103.15 ml (range of the means 93.9–160.5). After embolization, the residual decreased mainly in the first month to a pooled weighted mean of 66.56 ml (range of the means 61–72.3). The PVR decreased further to 57.88 ml at 12 months (range of the means 51.7–60.6). After 18 months follow-up, PVR started to increase to 88.0 ml (ranges of the means 74–93.54) at 24 months and 95.3 ml at 30 months in three patients. There was no significant effect on PVR in patients who underwent unilateral versus bilateral embolization, nor in patients who underwent embolization with 90–180 or 180–300 µm PVA particles [18, 19].
Qualitative clinical data All data on the IPSS, QOL, and international index of erectile dysfunction (IIEF) are presented in Tables 9, 10, 11.
IPSS The pooled weighted mean IPSS score at baseline was 23.31 (range of the means 21–24.7). After PAE, the IPSS decreased mainly in the first month to a score of 11.92 (range of the means 7.1–13.9). After the first month, the IPSS showed a further decrease to 8.1 at 30 months. The pooled weighted mean score at 36 months showed a slight increase to 9.1. There was no significant effect on IPSS score in patients who underwent unilateral versus bilateral embolization, nor in patients who underwent embolization with 90–180 or 180–300 µm PVA particles [18, 19].
QOL The pooled weighted mean QOL score at baseline was 4.34 (range of the means 3.86–6). After embolization, the QOL decreased mainly in the first month to a pooled weighted mean of 2.4 (range of the means 1.1–3.89). In the following months, the QOL showed a further decrease to 1.67 at 36 months. No significant effect on QOL score was stated in patients who underwent unilateral versus bilateral embolization or in patients who underwent embolization with 90–180 or 180–300 µm PVA particles [18, 19].
IIEF After PAE, no deterioration of erectile function was seen. After PAE, the IIEF score showed a maximum score of 20.58 at 6 months (range of the means 19.38–23.9); at baseline the pooled weighted mean IIEF score was 19.1 (range of the means 16.2–21.8). There was a significant greater score of IIEF when using 180–300 µm PVA particles compared with 90–180 µm particles (P = 0.043) [18].
Complications and other outcomes All complications are listed in detail per study in Supplement 2. Correct identification of the prostatic arteries is necessary to avoid untargeted ischemia to the bladder, rectum, anus, or corpus cavernosum [23]. Six cases of bladder ischemia were reported; of which two were transient, and four needed minor surgery, such as resection of a small area of necrosis. Transient rectal bleeding was reported in 20 cases, although no cases of intestinal wall ischemia were reported. No cases of ischemia of the corpus cavernosum were reported.
Most patients experienced no or mild pain. Only four patients experienced a lot of pain (VAS 9 or 10), related to four reported cases of bladder wall ischemia. A total of 21 patients experienced acute urinary retention after PAE, of which most were transient. Minor complications, such as hematoma on puncture site (n = 26), hematuria (n = 59), hematospermia (n = 38), urinary tract infection (n = 67), and prostatitis and balanitis (n = 10) were reported more frequently. However, these were transient or could be treated with antibiotics. No cases of impotence or retrograde ejaculation were reported. Most patients were discharged from the hospital on the day of the procedure (89 %). A minority was discharged the day after treatment (11 %) or 3 days after treatment (<1 %). Clinical failures after the first PAE were reported in a total of 131 patients, based on IPSS, QOL, and Qmax. Some of these patients underwent a second embolization procedure or a TURP.
Discussion
This review summarizes all the available evidence on PAE and all clinical outcomes were stated. The primary clinical outcomes seem positive. After PAE, a decrease of the PV and PVR was reported mainly in the first month with a further decrease up to 12 months, increasing afterwards. The PSA also decreased up to 3 months after PAE, increasing afterwards. The Qmax increased mainly the first month and decreased after 30 months. The IPSS and QOL improved mainly during the first month, with further improvement up to 30 months. No deterioration of IIEF was seen.
The PAE procedure seems safe. Only six patients had a transient or small area of bladder wall necrosis. These patients with bladder wall necrosis were presented in six different studies of two study groups [14–17, 20, 21]. There is probably an overlap in reported patients, but even than the complication rate is low. Although 20 cases of transient rectal bleeding were stated, no cases of intestinal ischemia were reported.
A major limitation of this review is that only a small number of studies (n = 9) are available and were published by only three different research groups; all are pioneers in the field of prostate embolization. The extent to which patients in different series overlap is unclear, but we assume that there is an overlap in patients and complications. Based on inclusion and exclusion criteria, inclusion period, and patients’ characteristics, we are certain that there were no full duplicates, because all presented data showed some differences in outcomes. Therefore, we decided to include all relevant papers.
The second limitation was the poor quality of studies, mainly based on the type of study (cohort), unclear patient selection, and dropouts. In the trials with more than 50 patients, a large dropout was already seen within 3 months, ranging from 6 to 48 % [15–17]. A follow-up of 18–36 months was only seen in three studies, representing a minority of patients [15–17]. In this small group of patients, a possible deterioration of parameters is seen. A relatively large number of clinical failures were described with persisting symptoms (131 patients; 19 %). Moreover, in none of the studies sample size calculations were given.
As for the methodological part of this systematic review, we did not perform a search in the Cochrane Library. However based on previous experience with at least 25 systematic reviews, we know that this database contains the same trials as Medline. As described earlier, different studies were reported by the same research group. Because we could not exclude duplication with certainty, we included all studies; our goal was to summarize the complete evidence on this topic. We therefore made several calculations to obtain means of mean values. We could not determine risk of bias across studies, in terms of whether good quality studies had other outcomes than poor quality studies, due to the low number of studies and overlap of data. This also is the reason that we did not perform a meta-analysis but downstaged this paper to a systematic review.
In the different studies, variations on embolization technique were described, using unilateral or bilateral embolization and using different embolization material. One study showed no significant differences in the quantitative or qualitative outcomes comparing unilateral versus bilateral PAE. However, they state a significantly greater chance of poor clinical outcome in the unilateral group (47.4 vs. 24.3 % in the bilateral group) [19]. Another study of the same research group compared PAE using 90–180 µm PVA particles with PAE using 180-300 µm PVA particles [18].
This study showed a significant lower PSA using the smaller particles. However, the IIEF score was significantly higher using larger particles. This is contradictory, because different particle sizes showed different benefits. However, the intention of the procedure is not to improve the IIEF score but to avoid deterioration. The ideal embolization technique should be explored in larger studies.
In conclusion, we state that the initial reported results of PAE seem promising, mainly during the first 12 months after treatment. However, no comparison was made to medical therapy or surgical therapies. Overlapping patient data and reporting bias could not be excluded. None of the included studies performed a power analysis. Also, a relatively small number of patients are treated with a short follow-up period. Therefore, more studies are needed with more patients and longer periods of follow-up, compared with standard medical and surgical therapies, to assess whether PAE is an effective and safe alternative treatment for BPH.
References
McVary KT (2006) BPH: epidemiology and comorbidities. Am J Manag Care 12(5 Suppl):S122–S128
Rosen RC, Giuliano F, Carson CC (2005) Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). Eur Urol 47(6):824–837
Wei JT, Calhoun E, Jacobsen SJ (2005) Urologic diseases in America project: benign prostatic hyperplasia. J Urol 173(4):1256–1261
Barry MJ, Fowler FJ Jr, O’Leary MP et al (1992) The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 148(5):1549–1557
O’Leary MP (2000) LUTS, ED, QOL: alphabet soup or real concerns to aging men? Urology 56(5 Suppl):7–11
American Urological Association Guideline: management of benign prostatic hyperplasia (BPH) (2010)
Michel MC, Mehlburger L, Bressel HU et al (1998) Tamsulosin treatment of 19,365 patients with lower urinary tract symptoms: does co-morbidity alter tolerability? J Urol 160(3 Pt 1):784–791
McConnell JD, Bruskewitz R, Walsh P et al (1998) The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med 338(9):557–563
Rassweiler J, Teber D, Kuntz R et al (2006) Complications of transurethral resection of the prostate (TURP)—incidence, management and prevention. Eur Urol 50(5):969–979
Sun F, Sánchez FM, Crisóstomo V et al (2008) Benign prostatic hyperplasia: transcatheter arterial embolization as potential treatment—preliminary study in pigs. Radiology 246(3):783–789
Jeon GS, Won JH, Lee BM et al (2009) The effect of transarterial prostate embolization in hormone-induced benign prostatic hyperplasia in dogs: a pilot study. J Vasc Interv Radiol 20(3):384–390
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Pisco JM, Pinheiro LC, Bilhim T et al (2011) Prostatic arterial embolization to treat benign prostatic hyperplasia. J Vasc Interv Radiol 22(1):11–19
Pisco JM, Rio Tinto H, Campos Pinheiro L et al (2013) Embolisation of prostatic arteries as treatment of moderate to severe lower urinary symptoms (LUTS) secondary to benign hyperplasia: results of short- and mid-term follow-up. Eur Radiol 23(9):2561–2572
Pisco J, Campos Pinheiro L, Bilhim T et al (2013) Prostatic arterial embolization for benign prostatic hyperplasia: short- and intermediate-term results. Radiology 266(2):668–677
Rio Tinto H, Martins Pisco J, Bilhim T et al (2012) Prostatic artery embolization in the treatment of benign prostatic hyperplasia: short and medium follow-up. Tech Vasc Interv Radiol 15(4):290–293
Bilhim T, Pisco J, Campos Pinheiro L et al (2013) Does polyvinyl alcohol particle size change the outcome of prostatic arterial embolization for benign prostatic hyperplasia? Results from a single-center randomized prospective study. J Vasc Interv Radiol 24(11):1595–1602
Bilhim T, Pisco J, Rio Tinto H et al (2013) Unilateral versus bilateral prostatic arterial embolization for lower urinary tract symptoms in patients with prostate enlargement. Cardiovasc Intervent Radiol 36(2):403–411
Carnevale FC, da Motta-Leal-Filho JM, Antunes AA et al (2013) Quality of life and clinical symptom improvement support prostatic artery embolization for patients with acute urinary retention caused by benign prostatic hyperplasia. J Vasc Interv Radiol 24(4):535–542
Antunes AA, Carnevale FC, da Motta Leal Filho JM et al (2013) Clinical, laboratorial, and urodynamic findings of prostatic artery embolization for the treatment of urinary retention related to benign prostatic hyperplasia. A prospective single-center pilot study. Cardiovasc Intervent Radiol 36(4):978–986
Bagla S, Martin CP, van Breda A et al (2014) Early results from a United States trial of prostatic artery embolization in the treatment of benign prostatic hyperplasia. J Vasc Interv Radiol 25(1):47–52 E-publication October 2013
Bilhim T, Tinto HR, Fernandes L et al (2012) Radiological anatomy of prostatic arteries. Tech Vasc Interv Radiol 15(4):276–285
Barry MJ (2001) Evaluation of symptoms and quality of life in men with benign prostatic hyperplasia. Urology 58(6 Suppl 1):25–32 discussion 32
O’Leary MP, Wei JT, Roehrborn CG et al (2008) Correlation of the International Prostate Symptom Score bother question with the Benign Prostatic Hyperplasia Impact Index in a clinical practice setting. BJU Int 101(12):1531–1535
Rosen RC, Cappelleri JC, Smith MD et al (1999) Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 11(6):319–326
Conflict of interest
Sanne M. Schreuder, Alexander E. Scholtens, Jim A. Reekers, and Shandra Bipat have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schreuder, S.M., Scholtens, A.E., Reekers, J.A. et al. The Role of Prostatic Arterial Embolization in Patients with Benign Prostatic Hyperplasia: A Systematic Review. Cardiovasc Intervent Radiol 37, 1198–1219 (2014). https://doi.org/10.1007/s00270-014-0948-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-014-0948-4