Abstract
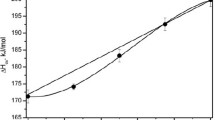
Structural parameters and cation ordering are determined for four compositions in the synthetic MgGa2O4-Mg2GeO4 spinel solid solution (0, 8, 15 and 23 mol% Mg2GeO4; 1400 °C, 1 bar) and for spinelloid β-Mg3Ga2GeO8 (1350 °C, 1 bar), by Rietveld refinement of room-temperature neutron diffraction data. Sample chemistry is determined by XRF and EPMA. Addition of Mg2GeO4 causes the cation distribution of the MgGa2O4 component to change from a disordered inverse distribution in end member MgGa2O4, [4]Ga = x = 0.88(3), through the random distribution, toward a normal cation distribution, x = 0.37(3), at 23 mol% Mg2GeO4. An increase in ao with increasing Mg2GeO4 component is correlated with an increase in the amount of Mg on the tetrahedral site, through substitution of 2 Ga3+⇄ Mg2++Ge4+. The spinel exhibits high configurational entropy, reaching 20.2 J mol−1 (four oxygen basis) near the compositional upper limit of the solid solution. This stabilizes the spinel in spite of positive enthalpy of disordering over the solid solution, where ΔH D = αx + βx 2, α = 22(3), β = −21(3) kJ mol−1. This model for the cation distribution across the join suggests that the empirically determined limit of the spinel solid solution is correlated with the limit of tetrahedral ordering of Mg, after which local charge-balanced substitution is no longer maintained.
Spinelloid β-Mg3Ga2GeO8 has cation distribution M1[Mg0.50(2)Ga0.50(2)] M2[Mg0.96(2)Ga0.04(2)] M3[Mg0.77(2) Ga0.23(2)]2 (Ge0.5Ga0.5)2O8 (tetrahedral site occupancies are assumed). Octahedral site size is correlated to Mg distribution, where site volume, site distortion, and Mg content follow the relation M1<M3<M2. The disordered cation distribution provides local electrical neutrality in the structure, and stabilization through increased configurational entropy (27.6 J mol−1; eight oxygen basis). Comparison of the crystal structures of Mg1+ N Ga2−2 N Ge N O4 spinel, β-Mg3Ga2GeO8, and Mg2GeO4 olivine reveals β-Mg3Ga2GeO8 to be a true structural intermediate. Phase transitions across the pseudobinary are necessary to accommodate an increasing divergence of cation size and valence, with addition of Mg2GeO4 component. Octahedral volume increases while tetrahedral volume decreases from spinel to β-Mg3Ga2GeO8 to olivine, with addition of Mg and Ge, respectively. Furthermore, M-M distances increase regularly across the join, suggesting that changes in topology reduce cation-cation repulsion.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 9 November 1998 / Revised, accepted: 3 August 1999
Rights and permissions
About this article
Cite this article
Millard, R., Peterson, R. & Swainson, I. Synthetic MgGa2O4-Mg2GeO4 spinel solid solution and β-Mg3Ga2GeO8: chemistry, crystal structures, cation ordering, and comparison to Mg2GeO4 olivine. Phys Chem Min 27, 179–193 (2000). https://doi.org/10.1007/s002690050006
Issue Date:
DOI: https://doi.org/10.1007/s002690050006