Abstract
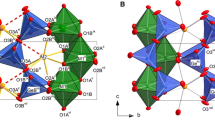
Triclinic Fe2+ and Fe3+-chloritoid end members, as well as Fe2+-Fe3+-chloritoid solid solutions were synthesized in order to determine the location of the OH groups in the structure and to investigate the possibility of Fe3+ incorporation on the M1B site and the corresponding oxidation mechanism. The samples were analyzed using transmission electron microscopy, electron energy-loss and 57Fe Mössbauer spectroscopy, polarized single-crystal, KBr powder and in situ high-pressure infrared spectroscopy. The structure of a natural triclinic chloritoid was refined by single-crystal X-ray diffraction in order to locate the proton-accepting oxygens in the triclinic structure. The results of valence bond calculations, polarized single-crystal and in situ high-pressure IR spectroscopy revealed that the orientations of the OH dipoles are the same as those in monoclinic chloritoid. The annealing experiments in air show that the incorporation of Fe3+ on M1B increases with temperature. After 3 h at 530 °C all Fe2+ was oxidized to Fe3+ without decomposition of the structure. This also holds true for a heating duration of 24 h. The incorporation of Fe3+ at M1B sites is coupled with a dehydration process starting at about 350 °C during which the H1A protons leave first.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 28 January 2000 / Accepted: 1 June 2000
Rights and permissions
About this article
Cite this article
Koch-Müller, M., Kahlenberg, V., Schmidt, C. et al. Location of OH groups and oxidation processes in triclinic chloritoid. Phys Chem Min 27, 703–712 (2000). https://doi.org/10.1007/s002690000114
Issue Date:
DOI: https://doi.org/10.1007/s002690000114