Abstract
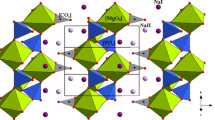
The compressibility and expansivity of anglesite (PbSO4) have been measured at high pressure up to 21.6 GPa and high temperature up to 700 K using in situ angle-dispersive X-ray diffraction and diamond anvil cell. The third-order Birch–Murnaghan equation of state (III-BM-EoS) was used to analyze the pressure–volume (P–V) data of PbSO4. We obtained the bulk modulus K0 = 59(1) GPa, and its pressure derivative \(K_{0}^{'}\) = 5.3(4), respectively. Using Holland–Powell thermal EoS to fit the temperature–volume (T–V) data, the thermal expansion coefficient α0 = 4.59(2) × 10− 5 K− 1 for PbSO4 was also derived. Simultaneously, the ambient-pressure axial compressibilities (βa0 = 1.79(4) × 10− 3 GPa− 1, βb0 = 1.75(5) × 10− 3 GPa− 1, βc0 = 2.12(4) × 10− 3 GPa− 1) and axial thermal expansivities (αa0 = 1.23(4) × 10− 5 K− 1, αb0 = 1.93(2) × 10− 5 K− 1, and αc0 = 1.43(1) × 10− 5 K− 1) along a-axis, b-axis and c-axis were derived at 300 K, respectively. Furthermore, the potential influencing factors (e.g., the effective size of M2+ cation, polarizability and electronegativity) on the bulk moduli of barite-type (belonging to Pbnm space group) sulfates (anglesite, barite, and celestine) were discussed. We found that the polarizability might be the most important factor. Finally, the anisotropic linear compressibility and thermal expansivity in barite-type sulfates were also discussed, respectively.










Similar content being viewed by others
References
Allen LC (1989) Electronegativity is the average one-electron energy of the valence-shell electrons in ground-state free atoms. J Am Chem Soc 111:9003–9014
Angel RJ (2000) Equations of state. Rev Miner Geochem 41:35–59
Angel RJ, Bujak M, Zhao J, Gatta GD, Jacobsen SD (2007) Effective hydrostatic limits of pressure media for high-pressure crystallographic studies. J Appl Crystallogr 40:26–32
Antao SM (2012a) The crystal structure of tin sulphate, SnSO4, and comparison with isostructural SrSO4, PbSO4, and BaSO4. Powder Diffr 27:179–183
Antao SM (2012b) Structural trends for celestite (SrSO4), anglesite (PbSO4), and barite (BaSO4): Confirmation of expected variations within the SO4 groups. Am Mineral 97:661–665
Birch F (1947) Finite elastic strain of cubic crystals. Phys Rev 71:809
Canfield DE (2004) The evolution of the Earth surface sulfur reservoir. Am J Sci 304:839–861
Canfield DE, Poulton SW, Narbonne GM (2007) Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science 315:92–95
Chen C, Liu L, Lin C, Yang Y (2001) High-pressure phase transformation in CaSO4. J Phys Chem Solids 62:1293–1298
Chen Y, Yu S, Huang E, Lee P (2010) Raman spectroscopy and X-ray diffraction studies on celestite. Phys B 405:4386–4388
Chen J, Yang R, Gao J, Zheng L, Du L, Yuan M, Wei H (2017) Mineralogy, sulfur isotopes and infrared microthermometric study of the Leishan-Rongjiang antimony ore field, SW China. Ac Geochim 36:339–352
Crichton WA, Parise JB, Antao SM, Grzechnik A (2005) Evidence for monazite-, barite-, and AgMnO4 (distorted barite)-type structures of CaSO4 at high pressure and temperature. Am Mineral 90:22–27
Crichton WA, Merlini M, Hanfland M, Müller H (2011) The crystal structure of barite, BaSO4, at high pressure. Am Mineral 96:364–367
Evans KA (2012) The redox budget of subduction zones. Earth-Sci Rev 113:11–32
Evans K, Tomkins A, Cliff J, Fiorentini M (2014) Insights into subduction zone sulfur recycling from isotopic analysis of eclogite-hosted sulfides. Chem Geol 365:1–19
Fan D, Zhou W, Wei S, Liu J, Li Y, Jiang S, Xie H (2010a) Phase relations and pressure-volume-temperature equation of state of galena. Chinese Phys Lett 27:086401
Fan D, Zhou W, Wei S, Liu Y, Ma M, Xie H (2010b) A simple external resistance heating diamond anvil cell and its application for synchrotron radiation X-ray diffraction. Rev Sci Instrum 81:053903
Fan D, Ma M, Wei S, Chen Z, Xie H (2013a) In-situ synchrotron powder X-ray diffraction study of vanadinite at room temperature and high pressure. High Temp-High Press 42:441–449
Fan D, Wei S, Liu J, Li Y, Xie H (2013b) X-ray diffraction study of calcium-lead fluorapatite solid solution at high pressure: the composition dependence of the bulk modulus and its pressure derivative. High Temp-High Press 42:69–80
Fan D, Xu J, Liu J, Li Y, Xie H (2014) Thermal equation of state of natural stibnite up to 25.7 GPa and 533 K. High Temp-High Press 43:351–359
Fan D, Xu J, Kuang Y, Li X, Li Y, Xie H (2015) Compressibility and equation of state of beryl (Be3Al2Si6O18) by using a diamond anvil cell and in situ synchrotron X-ray diffraction. Phys Chem Miner 42:529–539
Fei Y, Ricolleau A, Frank M, Mibe K, Shen G, Prakapenka V (2007) Toward an internally consistent pressure scale. Proc Natl Acad Sci USA 104:9182–9186
Fujii T, Ohfuji H, Inoue T (2016) Phase relation of CaSO4 at high pressure and temperature up to 90 GPa and 2300 K. Phys Chem Miner 43:353–361
Geng A, Ma Y, Li M, Li F, Cui Q (2009) Isothermal Equation of state and Raman spectroscopy of celestite. Chinese J At Mol Phys 26:753–756
Gonzalez-Platas J, Alvaro M, Nestola F, Angel R (2016) EosFit7-GUI: a new graphical user interface for equation of state calculations, analyses and teaching. J Appl Crystallogr 49:1377–1382
Gracia L, Beltrán A, Errandonea D, Andrés J (2011) CaSO4 and its pressure-induced phase transitions. A density functional theory study. Inorg Chem 51:1751–1759
Hammersley A, Svensson S, Hanfland M, Fitch A, Hausermann D (1996) Two-dimensional detector software: from real detector to idealised image or two-theta scan. Int J High Pressure Res 14:235–248
Hemley RJ, Zha CS, Jephcoat AP, Mao HK, Finger LW, Cox DE (1989) X-ray-diffraction and equation of state of sold neon to 110 GPa. Phys Rev B 39:11820–11827
Hilton DR, Fischer TP, Marty B (2002) Noble gases and volatile recycling at subduction zones. Rev Mineral Geochem 47:319–370
Holland T, Powell R (1998) An internally consistent thermodynamic data set for phases of petrological interest. J Metamorph Geol 16:309–343
James R, Wood W (1925) The crystal structure of barytes, celestine and anglesite. P Roy Soc A-Math Phy 109:598–620
Jégo S, Dasgupta R (2013) Fluid-present melting of sulfide-bearing ocean-crust: experimental constraints on the transport of sulfur from subducting slab to mantle wedge. Geochim Cosmochim Ac 110:106–134
Kuang Y, Xu J, Zhao D, Fan D, Li X, Zhou W, Xie H (2017) The high-pressure elastic properties of celestine and the high-pressure behavior of barite-type sulphates. High Temp-High Press 46:481–495
Larson AC, Von Dreele RB (2004) general structure analysis system (GSAS). Los Alamos Natl Lab LAUR 86–748:1–179
Le Bail A, Duroy H, Fourquet J (1988) Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater Res Bull 23:447–452
Lee P, Huang E, Yu S (2001) Phase diagram and equations of state of BaSO4. Int J High Pressure Res 21:67–77
Lee P, Huang E, Yu S (2003) High-pressure Raman and X-ray studies of barite, BaSO4. High Pressure Res 23:439–450
Lee P, Huang E, Yu S, Chen Y (2013) High-pressure Raman study on anglesite. World J Condens Matter Phys 3:28
Liu X, Shieh SR, Fleet ME, Akhmetov A (2008) High-pressure study on lead fluorapatite. Am Mineral 93:1581–1584
Liu J, Li S, Zhong J, Zhu X, Guo Q, Lang Y, Han X (2017) Sulfate sources constrained by sulfur and oxygen isotopic compositions in the upper reaches of the Xijiang River, China. Acta Geochim 36:611–618
Nie S, Liu Y, Liu Q, Wang M, Wang H (2017) Phase transitions and thermal expansion of BaCO3 and SrCO3 up to 1413 K. Eur J Miner 29:433–443
Pawley A, Redfern SA, Holland T (1996) Volume behavior of hydrous minerals at high pressure and temperature: I. Thermal expansion of lawsonite, zoisite, clinozoisite, and diaspore. Am Mineral 81:335–340
Pohl D (1978) Electronic polarizabilities of ions in doubly refracting crystals. Acta Crystallogr A 34:574–578
Richards JP (2011) Magmatic to hydrothermal metal fluxes in convergent and collided margins. Ore Geol Rev 40:1–26
Santamaría-Pérez D et al (2011) High-pressure study of the behavior of mineral barite by X-ray diffraction. Phys Rev B 84:054102
Sawada H, Takéuchi Y (1990) The crystal structure of barite, β-BaSO4, at high temperatures. ZKristallogr 191:161–171
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta crystallogr A 32:751–767
Toby BH (2001) EXPGUI, a graphical user interface for GSAS. J App Crystal 34:210–213
Tomkins AG, Evans KA (2015) Separate zones of sulfate and sulfide release from subducted mafic oceanic crust. Earth Planet Sci Lett 428:73–83
Webster J, Botcharnikov E R (2011) Distribution of sulfur between melt and fluid in S–O–H–C–Cl-bearing magmatic systems at shallow crustal pressures and temperatures. Rev Mineral Geochem 73:247–283
Xia X, Weidner J, Zhao D H (1998) Equation of state of brucite: single-crystal brillouin spectroscopy study and polycrystalline pressure-volume-temperature measurement. Am Mineral 83:68–74
Yang S, Zhong H, Zhu W, Hu W, Bai Z (2017) Platinum-group element geochemistry of mafic rocks from the Dongchuan area, southwestern China. Acta Geochim 36:52–65
Zhang J (1999) Room-temperature compressibilities of MnO and CdO: further examination of the role of cation type in bulk modulus systematics. Phys Chem Miner 26:644–648
Zhou L, Lee F, Wilcox W, Christensen J (2003) Magnetic polarizability of hadrons from lattice QCD. Nucl Phys B 119:272–274
Acknowledgements
This project was supported by the National Natural Science Foundation of China (Grant No. 41772043), the Joint Research Fund in Huge Scientific Equipment (U1632112) under cooperative agreement between NSFC and CAS, the Chinese Academy of Sciences “Light of West China” Program (Dawei Fan, 2017), Youth Innovation Promotion Association CAS (Dawei Fan, 2018), and the CPSF-CAS Joint Foundation for Excellent Postdoctoral Fellows (Grant No. 2017LH014). The high-pressure XRD experiments were performed at the High-Pressure Experiment Station (4W2), Beijing Synchrotron Radiation Facility (BSRF), and the BL15U1 of the Shanghai Synchrotron Radiation Facility (SSRF). We acknowledge H.Y. Su for the neon gas loading assistance. We would like to thank two anonymous reviewers for their thorough and helpful comments, which helped to improve the quality of this manuscript, and Professor Larissa Dobrzhinetskaya for handling this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, B., Xu, J., Chen, W. et al. Compressibility and expansivity of anglesite (PbSO4) using in situ synchrotron X-ray diffraction at high-pressure and high-temperature conditions. Phys Chem Minerals 45, 883–893 (2018). https://doi.org/10.1007/s00269-018-0970-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-018-0970-1