Abstract
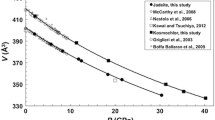
Kainite (KMg(SO4) Cl 3H2O) is a “mixed-salt” sulfate from the group of evaporitic minerals more soluble than Ca-sulfate hydrate and NaCl. The compressibility and structural modifications of monoclinic (sp. gr. C2/m) kainite up to a pressure of 14 GPa were studied by high-pressure single-crystal synchrotron X-ray diffraction. Kainite remains stable over the investigated pressure range and no phase transition was recognised. The bulk modulus is K0 = 31.6 (1) GPa, with K′ fixed to 4, as obtained by fitting the P-volume data with a second-order Birch–Murnaghan EoS (BM2); instead of using a BM3 EoS, we obtained K0 = 32.2(5) GPa, K’ =3.8 (1). The linear moduli calculated for the lattice parameters fitting the data with a BM3 EoS are for a-axis M0a = 117(4) GPa, Mpa = 11(1), for b-axis M0b = 113(2) GPa, Mpc = 8.6(5), and c-axis M0c = 68.2(3) GPa, Mpc = 14(1). Structure refinements showed a strong compression of the K polyhedra and in particular K(1) and K(3) polyhedra have similar polyhedral bulk moduli: K0K(1) = 20.8(7) GPa, K′=4.8(3); K0K(2) = 29(1) GPa, K′=8.1(6); K0K(3) = 26(1) GPa, K′=4.2(4). The most compressible bond distances are K(1)–Cl(2) with a shortening of about 13%, K(1)–Cl(1) with a shortening of about 10%, K(3)–Ow(6) and K(3)–O8(B) both with a shortening of 9%. S-tetrahedra are almost incompressible and Mg-octahedra bulk moduli are K0Mg(2) = 102(4) GPa, and K0Mg(4) = 72(1) GPa, K0Mg(1) = 41(4) GPa K′= 8.9(1.7), and K0Mg(3) = 65(5) GPa K′= 10(2). The strain tensor analysis indicates that the most compressible direction of the kainite monoclinic structure is oriented 29.7(2)° from the c-axis in the (0 1 0) plane. The shortening of the K(1)–K(2) distance (from 4.219(4) Å at ambient P to 3.521(7) Å at 11.9 GPa) and the different compressibilities of the octahedra/tetrahedra may explain why the stiffer direction of kainite is in the a–c plane approximatively along the direction where K(1)–K(2) and Mg(4)–Mg(3)–Mg(4) polyhedra align. This may explain the anisotropic compressional behaviour of the crystallographic axes, where c is more compressible (by tetrahedral tilting mechanism) than a and b, where cation–cation repulsion and a more rigid configuration make these directions stiffer. Following the structure modification increasing pressure a new sets of hydrogens bonds could form as oxygens and chlorine atoms get at less than 3 Å distance from the Ow.







Similar content being viewed by others
References
Angel RJ, Gonzalez-Platas J, Alvaro M (2014) EosFit7c and a Fortran module (library) for equation of state calculations. Z Kistallogr 229:405–419
Besson JM, Pinceaux JP (1979) Melting of helium at room temperature and high pressure. Science 206:1073–1075
Bish L, Scanlan MK (2006) The hydration and dehydration of hydrousmixed-cations. Lunar and Plan Sci XXXVII: 1011.pdf
Brown ID (1976) On the geometry of O-H… O hydrogen bonds. Acta Cryst A32:24–31
Comodi P, Fumagalli P, Montagnoli M, Zanazzi PF (2004) A single-crystal study on the pressure behavior of phlogopite and petrological implications. Am Mineral 89:647–653
Comodi P, Nazzareni S, Zanazzi PF, Speziale S (2008) High pressure behavior of gypsum: a single-crystal X-ray study. Am Mineral 93(10):1530–1537
Comodi P, Nazzareni S, Dubrovinsky L, Merlini M (2009) The high-pressure–high-temperature behavior of bassanite. Am Mineral 94(11–12):1596–1602
Comodi P, Kurnosov A, Nazzareni S, Dubrovinsky L (2011) The dehydration process of gypsum under high pressure. Phys Chem Min 39(1):65–71
Comodi P, Nazzareni S, Balic-Zunic T, Zucchini A, Hanfland M (2014) The high-pressure behavior of bloedite: A synchrotron single-crystal X-ray diffraction study. Am Mineral 99:511–518
Comodi P, Stagno V, Zucchini A, Fei Y, Prapapenka V (2016) The compression behavior of blödite at low and high temperature up to ~10 GPa: implications for the stability of hydrous sulfates on icy planetary bodies. Icarus 285:137–184
Fortes AD, Wood IG, Knight KS (2008) The crystal structure and thermal expansion tensor of MgSO4–11D2O(meridianiite) determined by neutron powder diffraction. Phys Chem Min 35(4):207–221
Gatta GD, Merlini M, Rotiroti N, Lotti P, Pavese A, Curetti N (2010) Structural evolution of a 2M(1) phengite mica up to 11 GPa: an in situ single-crystal X-ray diffraction study. Phys Chem Min 37(8):581–591
Gatta GD, Merlini M, Rotiroti N, Curetti N, Pavese A (2011) On the crystal chemistry and elastic behavior of a phlogopite 3T. Phys Chem Min 38(8):655–664
Gatta GD, Lotti P, Merlini M, Liermann HP, Lausi A, Valdre G, Pavese A (2015) Elastic behaviour and phase stability of pyrophyllite and talc at high pressure and temperature. Phys Chem Min 42(4):309–318
Hawthorne FC, Krivovichev SV, Burns PC (2000) The crystal chemistry of sulfate minerals. In: Alpens CN, Jambor JL, Nordstrom DK (eds) Sulfate minerals crystallography, geochemistry and environmental significance, 40. Rev Min Geochem, MSA, Chantilly, Virginia, pp 1–112
Ibers JA, Hamilton WC (eds) (1974) International Tables for X-ray Crystallography, vol 4:pp 99–101 Kinoch, Birmingham UK
King PL, Lescinsky DT, Nesbitt HW (2004) The composition and evolution of primordial solutions on Mars, with applications to other planetary bodies. Geochim Cosmochim Acta 68:4993–5008
Nakamura R, Ohtani E (2011) The high-pressure phase relation of the MgSO 4–H 2O system and its implication for the internal structure of Ganymede. Icarus 211(1):648–654
Nazzareni S, Comodi P, Bindi L, Dubrovinsky L (2010) The crystal structure of gypsum-II determined by single-crystal synchrotron X-ray diffraction data. Am Mineral 95(4):655–658
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic elongation, a quantitative measure of distortion in coordination polyhedra. Science 172:191–193
Robinson PD, Fang JH, Ohya Y (1972) The crystal structure of kainite. Am Mineral 57:1325–1332
Shannon RT, Prewitt CT (1969) Effective ionic radii in oxides and fluorides. Acta Cryst B 25:92
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A 64:112–122
Spencer RJ (2000) Sulfate minerals in evaporite deposits. In: Alpens CN, Jambor JL, Nordstrom DK (eds) Sulfate minerals Crystallography, Geochemistry and Environmental significance, 40. Rev Mineral Geochem, MSA, Chantilly, Virginia, pp 173–192
Syansson K (2008) Ruby under pressure. High Pres Res 28(2):75–126
Takemura K (2001) Evaluation of the hydrostaticity of a helium-pressure medium with powder X-ray diffraction techniques. J Appl Phys 89:662–668
Vaniman DT, Bish DL, Chipera SJ, Fialips CI, Carey JW, Feldman WC (2004) Magnesium sulphate salts and the history of water on Mars. Nature 431:663–665
Zanazzi PF, Montagnoli M, Nazzareni S, Comodi P (2006) Structural effects of pressure on triclinic chlorite: a single-crystal study. Am Mineral 91:1871–1878
Zanazzi PF, Montagnoli M, Nazzareni S, Comodi P (2007) Structural effects of pressure on monoclinic chlorite: A single-crystal study. Am Mineral 92:655–661
Zolotov MYu, Kuzmin RO, Shock EL (2004) Mineralogy, abundance, and hydration state of sulfates and chlorides at the mars pathfinder landing site. LPS XXXV:1465.pdf
Acknowledgements
M. Merlini is greatly acknowledged for its kindly assistance during the experiments at ID-09 beamline ESRF (Grenoble, France) and fruitful discussions. European Synchrotron Radiation Facility is acknowledged for allocating beam time (HS-4194). L. Bindi and the Mineralogical section of the Museo di Storia Naturale of Florence are kindly acknowledged for supplying the sample. Editor and reviewers are acknowledged for their suggestions that greatly improve the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nazzareni, S., Comodi, P. & Hanfland, M. High-pressure single-crystal synchrotron X-ray diffraction of kainite (KMg(SO4) Cl 3H2O). Phys Chem Minerals 45, 727–743 (2018). https://doi.org/10.1007/s00269-018-0958-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-018-0958-x