Abstract
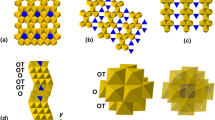
The crystal structure and chemical composition of a crystal of MgTiSi2O7 synthesized in the model system MgTiO3–MgSiO3 at 15 GPa and 1600 °C have been investigated. The compound was found to crystallize with the weberite-3T structure type, space group P3121, with lattice parameters a = 6.3351(7), c = 16.325(2) Å, V = 567.4(1) Å3, and Z = 6. The structure was refined to R 1 = 0.059 using 2092 independent reflections, and can be described as a sequence of pairs of polyhedral layers (M and N) stacked along [001]. As far as the cation sites are concerned, M and N layers have general formula AB3 and A3B, respectively, where B are the octahedrally coordinated cations (B1, B2, and B3), which mainly accommodate Si. The octahedral framework gives rise to three types of larger cavities occupied by eight-coordinated Mg (A1), Ti (A2), and mixed (Mg,Ti) (A3) atoms. Electron microprobe analysis gave the Mg0.99Si1.62Ti1.39O7 stoichiometry for the studied phase. The successful synthesis of this phase demonstrates that titanium can stabilize heretofore unknown Mg–Si-oxides, the major Earth and rocky planet-forming materials, and can provide new constraints on thermobarometry of wadsleyite/ringwoodite, and garnet-bearing assemblages.



Similar content being viewed by others
References
Albee AL, Ray L (1970) Correction factors for electron probe analysis of silicate, oxides, carbonates, phosphates, and sulfates. Anal Chem 48:1408–1414
Armstrong LS, Walter MJ, Tuff JR, Lord OT, Lennie AR, Kleppe AK, Clarke SM (2012) Perovskite phase relations in the system CaO-MgO-TiO2-SiO2 and implications for deep mantle lithologies. J Petrol 53:611–635
Bence AE, Albee AL (1968) Empirical correction factors for the electron microanalysis of silicate and oxides. J Geol 76:382–403
Bindi L, Sirotkina EA, Bobrov AV, Walter MJ, Pushcharovsky D, Irifune T (2017) Bridgmanite-like crystal structure in the novel Ti-rich phase synthesized at transition zone conditions. Am Miner 102:227–230
Cai L, Nino JC (2009) Complex ceramic structures. I. Weberites. Acta Crystallogr B 65:269–290
Coelho AA, Cheary RW, Smith KL (1997) Analysis and structural determination of Nd-substituted zirconolite-4M. J Solid State Chem 129:346–1359
Dobson DP, Jacobsen SD (2004) The flux growth of magnesium silicate perovskite single crystals. Am Miner 89:807–811
Fukui H, Yoneda A, Nakatsuka A, Tsujino N, Kamada S, Ohtani E, Shatskiy A, Hirao N, Tsutsui S, Uchiyama H, Baron AQR (2016) Effect of cation substitution on bridgmanite elasticity: a key to interpret seismic anomalies in the lower mantle. Sci Rep 6:33337
Grey IE, Mumme WG, Ness TJ, Roth RS, Smith KL (2003) Structural relations between weberite and zirconolite polytypes—refinements of doped 3 T and 4 M Ca2Ta2O7 and 3 T CaZrTi2O7. J Solid State Chem 174:285–295
Hill RJ, Newton MD, Gibbs GV (1983) A crystal chemical study of stishovite. J Solid State Chem 47:185–200
Ibers JA, Hamilton WC (eds) (1974) International tables for X-ray crystallography, vol. IV. Kynock, Dordrecht, p 366
Irifune T, Kurio A, Sakamoto S, Inoue T, Sumiya H, Funakoshi K (2004) Formation of pure polycrystalline diamond by direct conversion of graphite at high pressure and high temperature. Phys Earth Planet Inter 143–144:593–600
Ismailova L, Bykova E, Bykov M, Cerantola V, McCammon C, Boffa Ballaran T, Bobrov A, Sinmyo R, Dubrovinskaia N, Glazyrin K, Liermann H-P, Kupenko I, Hanfland M, Prescher C, Prakapenka V, Svitlyk V, Dubrovinsky L (2016) Stability of Fe, Al-bearing bridgmanite in the lower mantle and synthesis of pure Fe-bridgmanite. Sci Adv 2:e1600427
Katsura T, Ito E (1989) The system Mg2SiO4-Fe2SiO4 at high pressure and temperatures: precise determination of stabilities of olivine, modified spinel, and spinel. J Geophys Res 94:15663–15670
Nishio-Hamane D, Yagi T, Ohshiro M, Niwa K, Okada T, Seto Y (2010) Decomposition of perovskite FeTiO3 into wüstite Fe1–x Ti0.5x O and orthorhombic FeTi3O7 at high pressure. Phys Rev B 82:092103
Nishio-Hamane D, Zhang M, Yagi T, Ma Y (2012) High-pressure and high-temperature phase transitions in FeTiO3 and a new dense FeTi3O7 structure. Am Miner 97:568–572
Niu H, Oganov A, Chen X-Q, Li D (2015) Prediction of novel stable compounds in the Mg-Si-O system under exoplanet pressures. Sci Rep 5:18347.
Oxford Diffraction (2006) CrysAlis RED (Version 1.171.31.2) and ABSPACK in CrysAlis RED. Oxford Diffraction Ltd, Abingdon
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic elongation: a quantitative measure of distortion in coordination polyhedra. Science 172:567–570
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32:751–767
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122
Sirotkina EA, Bobrov AV, Bindi L, Irifune T (2015) Phase relations and formation of chromium-rich phases in the system Mg4Si4O12–Mg3Cr2Si3O12 at 10–24 GPa and 1600 °C. Contr Mineral Petrol. doi:10.1007/s00410-014-1097-0
Yamada A, Inoue T, Irifune T (2004) Melting of enstatite from 13 to 18 GPa under hydrous conditions. Phys Earth Planet Inter 147:45–56
Acknowledgements
The research was supported by “progetto di Ateneo 2015, University of Firenze” to LB, by C.N.R., Istituto di Geoscienze e Georisorse sezione di Firenze, Italy, by the Foundation of the President of the Russian Federation (grant no. MK-1277.2017.5 to ES), and by the Russian Foundation for Basic Research (project no. 15-05-02051 to DP). ES thanks Geodynamics Research Center, Ehime University, Matsuyama, Japan, for support of her visit in 2016.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bindi, L., Sirotkina, E.A., Bobrov, A.V. et al. Discovery of MgTiSi2O7: a new high-pressure silicate with the weberite structure synthesized at transition-zone conditions. Phys Chem Minerals 44, 419–424 (2017). https://doi.org/10.1007/s00269-016-0868-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-016-0868-8