Abstract
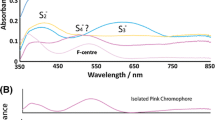
Several samples of wulfenite, PbMoO4, varying in colour from colourless to yellow, orange and red, have been characterised by means of IR and optical absorption spectroscopy and by microprobe analyses. A distinct pleochroic band group with absorption maxima centred at 3,380 and 3,150 cm−1 can be seen in the IR spectra of wulfenite single-crystals, indicating the presence of hydroxyl groups. The pleochroic and thermal behaviour of the OH stretching bands along with deuteration experiments, as well as results obtained from synthetic flux-grown samples, exclude the presence of submicroscopic hydrous mineral inclusions as their primary origin. The pleochroic scheme and the band positions were used to postulate a model for the OH incorporation mode, based on the assumption of vacancies on Mo and Pb sites in the structure of this ‘nominally anhydrous mineral’. Optical absorption spectra of coloured natural samples show a broad and polarised band around 23,000–24,000 cm−1, preceding the fundamental UV absorption edge, which has been identified as the reason for the colour of the mineral. The comparison with synthetic PbMoO4 single-crystals, doped with variable amounts of Cr6+, yielded conclusive evidence that trace amounts of the CrO4 2− anion group, substituting for MoO4 2−, determine the variable colour. Besides, in one sample, trace amounts of Nd3+ have been spectroscopically identified.












Similar content being viewed by others
References
Andrut M, Brandstätter F, Beran A (2003) Trace hydrogen zoning in diopside. Miner Petrol 78:231–241
Beran A, Rossman GR (2006) OH in naturally occurring corundum. Eur J Miner 18:441–447
Beran A, Langer K, Andrut M (1993) Single crystal infrared spectra in the range of OH fundamentals of paragenetic garnet, omphacite and kyanite in an eclogitic mantle xenolith. Miner Petrol 48:257–268
Beran A, Talla D, Losos Z, Pinkas J (2010) Traces of structural H2O molecules in baryte. Phys Chem Miner 37:159–166
Bollmann W (1980) Coloration, photoconductivity, photo- and thermoluminescence of PbMoO4 crystals. Kristall und Technik 15:367–375
Downs RT (2006) The RRUFF project: an integrated study of the chemistry, crystallography, Raman and infrared spectroscopy of minerals. Program and Abstracts of the 19th General Meeting of the IMA in Kobe, Japan: O03–13
Dutrizac JE (1983) Factors affecting alkali jarosite precipitation. Metall Trans B 14B:531–539
Fujita M, Itoh M, Mitani H, Sangeeta S, Tyagi M (2010) Exciton transition and electronic structure of PbMoO4 crystals studied by polarized light. Phys Stat Sol B 247:405–410
Haberlandt H, Schroll E (1950) Färbung und Fluoreszenz des Wulfenites im Zusammenhang mit dem Gehalt an Chrom und anderen Spurenelementen. Experientia (now: Cellular and Molecular Life Sciences) 6:89–91
Hales MC, Frost RL (2007) Synthesis and vibrational spectroscopic characterisation of synthetic hydrozincite and smithsonite. Polyhedron 26:4955–4962
Ikornikova NY, Sheptunov VM (1973) Dissociation curves of trigonal carbonates. In: Lobachev AN (ed) Crystallization processes under hydrothermal conditions. Consultants Bureau, New York
Johnson EA (2006) Water in nominally anhydrous crustal minerals: speciation, concentration, and geologic significance. In: Keppler H, Smyth JR (eds) Water in nominally anhydrous minerals. Rev Mineral Geochem 62:117–154
Keller P (1984) Tsumeb. Lapis 9:13–63
Khisina NR, Wirth R, Andrut M, Ukhanov AV (2001) Extrinsic and intrinsic mode of hydrogen occurrence in natural olivines: FTIR and TEM investigation. Phys Chem Miner 28:291–301
Koch-Müller M, Rhede D (2010) IR absorption coefficients for water in nominally anhydrous high-pressure minerals. Am Miner 95:770–775
Libowitzky E (1999) Correlation of O-H stretching frequencies and O-H···O hydrogen bond lengths in minerals. Mh Chemie 130:1047–1059
Libowitzky E, Beran A (2004) IR spectroscopic characterisation of hydrous species in minerals. In: Beran A, Libowitzky E (eds) Spectroscopic methods in mineralogy. EMU Notes Miner 6:227–279
Libowitzky E, Beran A (2006) The structure of hydrous species in nominally anhydrous minerals: information from polarized IR spectroscopy. In: Keppler H, Smyth JR (eds) Water in nominally anhydrous minerals. Rev Miner Geochem 62:29–52
Libowitzky E, Rossman GR (1996) Principles of quantitative absorbance measurements in anisotropic crystals. Phys Chem Miner 23:319–327
Libowitzky E, Rossman GR (1997) An IR absorption calibration for water in minerals. Am Miner 82:1111–1115
Libowitzky E, Beran A, Wieczorek A, Wirth R (2012) On the presence of a hydrous component in a gemstone variety of intermediate olivine-type triphylite-lithiophilite, Li(Fe, Mn)PO4. Miner Petrol 105:31–39
Liu Q-J, Liu Z-T, Feng L-P, Tian H (2011) First-principles study of electronic structure and optical properties of tetragonal PbMoO4. ISRN Cond Matter Phys 2011:id290741
Lugli C, Medici L, Saccardo D (1999) Natural wulfenite: structural refinement by single- crystal X-ray diffraction. N Jb Miner Mh 1999:281–288
Matsyuk SS, Langer K (2004) Hydroxyl in olivines from mantle xenoliths in kimberlites of the Siberian platform. Contr Miner Petrol 147:413–437
Mikenda W (1986) Stretching frequency versus bond distance correlation of O-D(H)…Y (Y = N, O, S, Se, Cl, Br, I) hydrogen bonds in solid hydrates. J Mol Str 147:1–15
Miller GH, Rossman GR, Harlow GE (1987) The natural occurrence of hydroxide in olivine. Phys Chem Miner 14:461–472
Minhas IS, Sharma KK, Gruber JB (1973) Optical absorption and Zeeman spectra of Nd3+-doped PbMnO4. Phys Rev B 8:385–392
Mosenfelder JL, Le Voyer M, Rossman GR, Guan Y, Bell DR, Asimov PD, Eiler JM (2011) Analysis of hydrogen in olivine by SIMS: evaluation of standards and protocol. Am Miner 96:1725–1741
Novak A (1974) Hydrogen bonding in solids: correlation of spectroscopic and crystallographic data. Struct Bond 18:177–216
Parant JP, Villela G, Gourier D, Le Sergent C, Dumas JP (1981) Influence of chromium content on the coloration of PbMoO4 crystals. J Cryst Growth 52:576–579
Paterson MS (1982) The determination of hydroxyl by infrared absorption in quartz silicate glasses and similar materials. Bull Miner 105:20–29
Rossman GR (2006) Analytical methods for measuring water in nominally anhydrous minerals. In: Keppler H, Smyth JR (eds) Water in nominally anhydrous minerals. Rev Miner Geochem 62:1–28
Ryskin YI (1974) The vibrations of protons in minerals: hydroxyl, water and ammonium. In: Farmer VC (ed) The infrared spectra of minerals. Mineral Soc, London, pp 137–181
Schwarz D, Pardieu V, Saul JM, Schmetzer K, Laurs BM, Giuliani G, Klemm L, Malsy A-K, Erel E, Hauzenberger C, Du Toit G, Fallick AE, Ohnenstetter D (2008) Rubies and sapphires from Winza, Central Tanzania. Gems Gemology 44:322–347
Shimodaira Y, Kato H, Kobayashi H, Kudo A (2007) Investigations of electronic structures and photocatalytic activities under visible light irradiation of lead molybdate replaced with chromium(VI). Bull Chem Soc Jpn 80:885–893
Skogby H, Rossman GR (1989) OH− in pyroxene: an experimental study of incorporation mechanisms and stability. Am Miner 74:1059–1069
Talla D, Beran A, Skoda R, Losos Z (2011) On the presence of OH defects in the zircon-type phosphate mineral xenotime, (Y, REE)PO4. Am Miner 96:1799–1808
Tyagi M, Singh SG, Singh AK, Gadkari SC (2010) Understanding colorations in PbMoO4 crystals through stoichiometric variations and annealing studies. Phys Stat Sol A 207:1802–1806
Wieczorek A, Libowitzky E, Beran A (2004) A model for the OH defect incorporation in kyanite based on polarised IR spectroscopic investigations. Schweiz Min Petr Mitt 84:333–343
Zeng HC (1996) Synthesis of stoichiometric lead molybdate PbMoO4: an X-ray diffraction, Fourier transform infrared spectroscopy, and differential thermal analysis study. J Mater Res 11:703–715
Zhang M, Moxon T (2011) In situ infrared spectroscopic studies of OH, H2O and CO2 in moganite at high temperatures. Eur J Miner 24:123–131
Zhang M, Salje EKH, Ewing RC (2010) OH species, U ions, and CO/CO2 in thermally annealed metamict zircon (ZrSiO4). Am Miner 95:1717–1724
Acknowledgments
Samples were kindly provided by the mineral collection of the ‘Institut für Mineralogie und Kristallographie, Universität Wien’. Thanks are due to A. Wagner for careful sample preparation. D.T. acknowledges the award of a scholarship by the Austrian Federal Ministry of Science and Research within the frame of the Austria Exchange Service (ÖAD), Academic Mobility Unit ACM-2008-00061 and the award of a CEEPUS scholarship (CIII-RO-0038-1213). This work was supported by the projects ‘CEITEC-Central European Institute of Technology’ (CZ.1.05/1.1.00/02.0068) and GACR P207/11/0555 of the Grant Academy of the Czech Republic. We thank Henrik Skogby and Monika Koch-Müller for their reviews, which helped to significantly improve the quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talla, D., Wildner, M., Beran, A. et al. On the presence of hydrous defects in differently coloured wulfenites (PbMoO4): an infrared and optical spectroscopic study. Phys Chem Minerals 40, 757–769 (2013). https://doi.org/10.1007/s00269-013-0610-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-013-0610-8