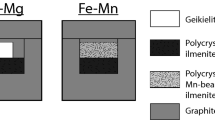
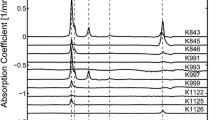
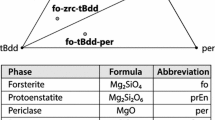
Abstract
The interdiffusion coefficient of Mg–Fe in olivine (D Mg–Fe) was obtained at 1,400–1,600 °C at the atmospheric pressure with the oxygen fugacity of 10−3.5–10−2 Pa using a diffusion couple technique. The D Mg–Fe shows the anisotropy (largest along the [001] direction and smallest along the [100] direction), and its activation energy (280–320 kJ/mol) is ~80–120 kJ/mol higher than that estimated at lower temperatures. The D Mg–Fe at temperatures of >1,400 °C can be explained by the cation-vacancy chemistry determined both by the Fe3+/Fe2+ equilibrium and by the intrinsic point defect formation with the formation enthalpy of 220–270 kJ/mol depending on the thermodynamical model for the Fe3+/Fe2+ equilibrium in olivine. The formation enthalpy of 220–270 kJ/mol for the point defect (cation vacancy) in olivine is consistent with that estimated from the Mg self-diffusion in Fe-free forsterite. The increase in the activation energy of D Mg–Fe at >1,400 °C is thus interpreted as the result of the transition of diffusion mechanism from the transition metal extrinsic domain to the intrinsic domain at the atmospheric pressure.








Similar content being viewed by others
References
Buening DK, Buseck PR (1973) Fe–Mg lattice diffusion in olivine. J Geophys Res 78:6852–6862
Chakraborty S, Farvet JR, Yund RA, Rubie DC (1994) Mg tracer diffusion in synthetic forsterite and San Carlos olivine as a function of P, T, and fO2. Phys Chem Miner 21:489–500
Chakraborty S (1997) Rates and mechanisms of Fe–Mg interdiffusion in olivine at 980°C–1300°C. J Geophys Res 102(B6):12317–12331
Chakraborty S (2010) Diffusion coefficients in olivine, wadsleyite and ringwoodite. In: Zhang Y, Cherniak DJ (eds) Reviews in mineralogy and geochemistry, vol 72. Mineralogical Society of America, Chantilly, Virginia, pp 603–639
Chatterjee S, Saha-Dasgupta T (2010) First-principles simulations of structural, electronic, and magnetic properties of vacancy-bearing Fe silicates. Phys Rev B 81:155105
Chatterjee S, Sengupta S, Saha-Dasgupta T, Chatterjee K, Mandal N (2009) Site preference of Fe atoms in FeMgSiO4 and FeMg(SiO3)2 studied by density functional calculations. Phys Rev B 79:115103
Chatterjee S, Bhattacharyya S, Sengupta S, Saha-Dasgupta T (2011) Crossover of cation partitioning in olivines: a combination of ab initio and Monte Carlo study. Phys Chem Miner 38:259–265
Dohmen R, Chakraborty S (2007) Fe-Mg diffusion in olivine II: point defect chemistry, change of diffusion mechanisms and a model for calculation of diffusion coefficients in natural olivine. Phys Chem Miner 34:409–430
Dohmen R, Becker HW, Chakraborty S (2007) Fe-Mg diffusion in olivine I: experimental determination between 700 and 1,200 degrees C as a function of composition, crystal orientation and oxygen fugacity. Phys Chem Miner 34:389–407
Düffels K, Chakraborty S, Brenker FE (2004) Enhacement of diffusion rates in olivine during evaporation—an example of reactive diffusion in a mineralogical system. Lithos 73:S30
Hashimoto A (1983) Evaporation kinetics of forsterite and implication for the early solar nebula. Nature 347:53–55
Hewins RH, Ganguly J, Mariani E (2009) Diffusion modeling of cooling rates of relict olivine in Semarkona chondrules (abstract). Lunar Planet Sci Abstract 40:#1531
Holzapfel C, Chakraborty S, Rubie DC, Frost DJ (2007) Effect of pressure on Fe–Mg, Ni and Mn diffusion in (FexMg1−x)2SiO4 olivine. Phys Earth Planet Inter 162:186–198
Jurewicz AJG, Watson EB (1988) Cation in olivine, Part 1: calcium partitioning and calcium–magnesium distribution between olivines and coexisting melts, with petrologic applications. Contrib Mineral Petrol 99:176–185
Kuroda D, Hashimoto A (2002) The reaction of forsterite with hydrogen—its apparent and real temperature dependences. Antarct Met Res 15:152–164
Lasaga AC (1980) Defect calculation in silicates: olivine. Am Miner 65:1237–1248
Matsumoto N, Nagahara H, Ozawa K, Tachibana S, Kawasaki H, Tamada S (2006) Evaporation anisotropy of olivine. In: 19th International mineral association. Abstract 90869
Misener DJ (1974) Cation diffusion in olivine to 1400°C and 35 kbar. In: Hofmann AW, Giletti BJ, Yoder HS Jr, Yund RA (eds) Geochemical transport and kinetics, vol 634. Carnegie Inst. Wash. Publ., Washington Carnegie Inst., Washington, pp 117–129
Miyamoto M, Mikouchi T, Jones RH (2009) Cooling rates of porphyritic olivine chondrules in the Semarkona (LL3.00) ordinary chondrite: a model for diffusional equilibration of olivine during fractional crystallization. Meteorit Planet Sci 44:521–530
Morioka M (1980) Cation diffusion in olivine—I. Cobalt and magnesium. Geochim Cosmochim Acta 44:759–762
Mueller T (2010) Applications of diffusion data to high-temperature Earth systems. In: Zhang Y, Cherniak DJ (eds) Reviews in mineralogy and geochemistry, vol 72. Mineralogical Society of America, Chantilly, Virginia, pp 997–1038
Nagahara H, Ozawa K (1996) Evaporation of forsterite in H2 gas. Geochim Cosmochim Acta 60:1445–1459
Nakamura A, Schmalzried H (1983) On the nonstoichiometry and point defects of olivine. Phys Chem Miner 10:27–37
Nakamura A, Schmalzried H (1984) On the Fe2+–Mg2+-interdiffusion in olivine (II). Ber Bunsenges Phys Chem 88:140–145
Ozawa K (1984) Olivine-spinel geospeedometry: analysis of diffusion-controlled Mg–Fe2+ exchange. Geochim Cosmochim Acta 48:2597–2611
Ozawa K, Nagahara H (2000) Kinetics of diffusion-controlled evaporation of Fe–Mg olivine: experimental study and implication for stability of Fe-rich olivine in the solar nebula. Geochim Cosmochim Acta 64:939–955
Ozawa K, Nagahara H, Morioka M, Matsumoto N, Hutcheon ID, Noguchi T, Kagi H (2012) Kinetics of evaporation of forsterite in vacuum. Amer Mineral 97:80–99
Petry C, Chakraborty S, Palme H (2004) Experimental determination of Ni diffusion coefficients in olivine and their dependence on temperature, composition, oxygen fugacity, and crystallographic orientation. Geochim Cosmochim Acta 68:4179–4188
Smyth JR, Frost DJ, Nestola F, Holl CM, Bromiley G (2006) Olivine hydration in the deep upper mantle: effects of temperature and silica activity. Geophys Res Lett 33:L15301. doi:10.1029/2006GL026194
Stocker RL, Smyth JR (1978) Effect of enstatite activity and oxygen partial pressure on the point defect chemistry of olivine. Phys Earth Planet Inter 16:145–156
Takigawa A, Tachibana S, Nagahara H, Ozawa K, Yokoyama M (2009) Anisotropic evaporation of forsterite and its implication for dust formation conditions in circumstellar environments. Astrophys J 707:L97–L101
Taylor L, Onorato PIK, Uhlmann DR (1977) Cooling rate estimations based on kinetic modeling of Fe-Mg diffusion in olivine. Proc Lunar Sci Conf 8:1581–1592
Tsai T-L, Dieckmann R (2002) Variation of the oxygen content and point defects in olivines (FexMg1–x)2SiO4, 0.2 ≤ x ≤ 1.0. Phys Chem Miner 29:680–694
Tsuchiyama A, Takahashi T, Tachibana S (1998) Evaporation rates of forsterite in the system Mg2SiO4−–H2. Mineral J 20:113–126
Walker D, Kirkpatrick RJ, Longhi J, Hays JF (1976) Crystallization of lunar picritic basalt sample 12002: phase equilibria and cooling rate studies. Bull Geol Soc Am 87:646–656
Walker D, Longhi J, Stolper EM, Grove TL, Hays JF (1977) Slowly cooled microgabbros 15065 and 15555 (abstract). Lunar Sci Conf VIII:964–966
Walker AM, Woodley SM, Slater B, Wright K (2009) A computational study of magnesium point defects and diffusion in forsterite. Phys Earth Planet Inter 172:20–27
Wang J, Davis AM, Clayton RN, Hashimoto A (1999) Evaporation of single crystal forsterite: evaporation kinetics, magnesium isotope fractionation, and implications of mass-dependent isotopic fractionaion of a diffusion-controlled reservoir. Geochim Cosmochim Acta 63:953–966
Yamada M, Tachibana S, Nagahara H, Ozawa K (2006) Anisotropy of Mg isotopic fractionation during evaporation and Mg self-diffusion of forsterite in vacuum. Planet Space Sci 54:1096–1106
Yoon DN, Lazarus D (1972) Pressure dependence of ionic conductivity in KCl, NaCl, KBr, NaBr. Phys Rev B 5:4935–4945
Zhao YH, Ginsberg SB, Kohlstedt DL (2004) Solubility of hydrogen in olivine: dependence on temperature and iron content. Contrib Mineral Petrol 147:155–161
Acknowledgments
We would like to thank Masana Morioka for his assistance in synthesizing single crystals of Fe-free forsterite. We would also like to thank Hideto Yoshida fro his help in electron microprobe analyses. Careful reviews by Sumit Chakraborty, an anonymous reviewer, and Editor Masanori Matsui are appreciated, which made the quality of the paper much improved. This work was partly supported by Grant-in-Aid for Scientific Research (S) (16104007) and Grant-in-Aids for Young Scientists (A) (20684025).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tachibana, S., Tamada, S., Kawasaki, H. et al. Interdiffusion of Mg–Fe in olivine at 1,400–1,600 °C and 1 atm total pressure. Phys Chem Minerals 40, 511–519 (2013). https://doi.org/10.1007/s00269-013-0588-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-013-0588-2