Abstract
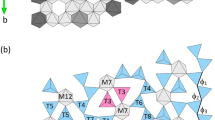
Synthetic melilites on the join Ca2MgSi2O7 (åkermanite: Ak)-Ca2Fe3+AlSiO7 (ferrialuminium gehlenite: FAGeh) were studied using X-ray powder diffraction and 57Fe Mössbauer spectroscopic methods to determine the distribution of Fe3+ between two different tetrahedral sites (T1 and T2), and the relationship between ionic substitution and incommensurate (IC) structure. Melilites were synthesized from starting materials with compositions of Ak100, Ak80FAGeh20, Ak70FAGeh30 and Ak50FAGeh50 by sintering at 1,170–1,350 °C and 1 atm. The average chemical compositions and end-member components, Ak, FAGeh and Geh (Ca2Al2SiO7), of the synthetic melilites were Ca2.015Mg1.023Si1.981O7 (Ak100), Ca2.017Mg0.788Fe 3+0.187 Al0.221Si1.791O7 (Ak78FAGeh19Geh3), Ca1.995Mg0.695Fe 3+0.258 Al0.318Si1.723O7 (Ak69FAGeh25Geh6) and Ca1.982Mg0.495Fe 3+0.449 Al0.519Si1.535O7 (Ak49FAGeh44Geh7), respectively. Rietveld refinements using X-ray powder diffraction data measured using CuK α -radiation at room temperature converged successfully with goodness-of-fits of 1.15–1.26. The refined Fe occupancies at the T1 and T2 sites and the Mg and Si contents determined by electron microprobe analysis gave the site populations of [0.788Mg + 0.082Fe3+ + 0.130Al]T1[0.104Fe3+ + 0.104Al + 1.792Si]T2 for Ak78FAGeh19Geh3, [0.695Mg + 0.127Fe3+ + 0.178Al]T1[0.132Fe3+ + 0.144Al + 1.724Si]T2 for Ak69FAGeh25Geh6 and [0.495Mg + 0.202Fe3+ + 0.303Al]T1[0.248Fe3+ + 0.216Al + 1.536Si]T2 for Ak49FAGeh44Geh7 (apfu: atoms per formula unit), respectively. The results indicate that Fe3+ is distributed at both the T1 and the T2 sites. The mean T1–O distance decreases with the substitution of Fe3+ + Al3+ for Mg2+ at the T1 site, whereas the mean T2–O distance increases with substitution of Fe3+ + Al3+ for Si4+ at the T2 site, causing decrease in the a dimension and increase in the c dimension. However, in spite of the successful Rietveld refinements for the X-ray powder diffraction data measured using CuK α-radiation at room temperature, each Bragg reflection measured using CuK α1-radiation at room temperature showed weak shoulders, which were not observed in those measured at 200 °C. The Mössbauer spectra of the melilites measured at room temperature consist of two doublets assigned to Fe3+ at the T1 site and two or three doublets to Fe3+ at the T2 site, implying the existence of multiple T1 and T2 sites with different site distortions. These facts can be interpreted in terms of the IC structure in all synthetic melilites at room temperature, respectively. The results of Mössbauer analysis indicate that the IC structure in melilite is caused by not only known multiple T1 site, but also multiple T2 site at room temperature.






Similar content being viewed by others
References
Akasaka M, Ohashi H (1985) 57Fe Mössbauer study of synthetic Fe3+-melilites. Phys Chem Miner 12:13–18
Akasaka M, Shinno I (1992) Mössbauer spectroscopy and its recent application to silicate mineralogy. Miner J 21:3–20
Akasaka M, Ohashi O, Shinno I (1986) The distribution of Fe3+ and Ga3+ between two tetrahedral sites in melilites, Ca2(Mg, Fe3+, Ga, Si)3O7. Phys Chem Miner 13:152–155
Akasaka M, Nagashima M, Makino K, Ohashi H (2005) Distribution of Fe3+ in a synthetic (Ca,Na)2(Mg,Fe3+)Si2O7-melilite: 57Fe Mössbauer and X-ray Rietveld studies. J Miner Petrol Sci 100:229–236
Ardit M, Dondi M, Merlini M, Cruciani G (2012) Melilite-type and melilite-related compounds: structural variations along the join Sr2-x Ba x MgSi2O7 (0 ≤ x ≤ 2) and high-pressure behavior of the two end-members. Phys Chem Miner 39:199–211
Armbruster T, Röthlisberger F, Seifert F (1990) Layer topology, stacking variation, and site distortion in melilite-related compounds in the system CaO–ZnO–GeO2–SiO2. Am Miner 75:847–858
Bancroft GM (1973) Mössbauer spectroscopy: an introduction for inorganic chemists and geochemists. Wiley, New York p 251
Bindi L, Bonazzi P (2005) IC-normal phase transition in natural melilite: an in situ high-temperature X-ray single-crystal study. Phys Chem Miner 32:89–96
Brese NE, O’Keeffe M (1991) Bond–valence parameters for solids. Acta Crystallogr B47:192–197
Brown ID, Altermatt D (1985) Bond-valence parameters obtained from a systematic analysis of the Inorgarnic crystal structure database. Acta Crystallogr A29:266–282
Dollase WA (1986) Correction of intensities for preferred orientation in powder diffractometry: application of the March model. J App Crystallogr 19:267–272
Fitton JG, Hughes DJ (1981) Strontian melilite in a nephelinite lava from Etinde, Cameroon. Miner Mag 44:261–264
Hamada M (2011) Sr-Na-bearing åkermanite and nepheline in nephelinite from Nagahama, Hamada, Shimane Prefecture, Southwest Japan. J Miner Petrol Sci 106:187–194
Hemingway BS, Evans HT Jr, Nord GL Jr, Haselton HT Jr, Robie RA, McGee JJ (1986) A study of phase transitions in the heat capacity and thermal expansion of åkermanite, Ca2MgSi2O7, and revised values for the thermodynamic properties of åkermanite. Can Miner 24:425–434
Hill RJ, Flack HD (1987) The use of the Durbin-Watson d statistic in Rietveld analysis. J App Crystallogr 20:356–361
Huckenholz HG, Ott OD (1978) Synthesis, stability, and aluminum-rion substitution in gehlenite-ferrigehlenite solid solutions. Neues Jahrb Miner Mon H.2:83–96
Izumi F (1993) Rietveld analysis program RIETAN and PREMOS and special applications. In: Young RA (ed) The rietveld method. Oxford Science Publications, Oxford, pp 236–253
Izumi F, Momma K (2007) Three-dimensional visualization in powder diffraction. Solid State Phenom 130:15–20
Kimata M (1983a) The crystal structure and stability of Co-åkermanite, Ca2CoSi2O7, compared with the mineralogical behavior of Mg cation. Neues Jahrb Miner Abh 146:221–241
Kimata M (1983b) The structural properties of synthetic Sr-åkermanite, Sr2MgSi2O7. Z Kristallogr 163:295–304
Kimata M, Ii N (1981) The crystal structure of synthetic åkermanite, Ca2MgSi2O7. Neues Jahrb Miner Abh 15:1–10
Kimata M, Ii N (1982) The structural property of synthetic gehlenite, Ca2Al2SiO7. Neues Jahrb Miner Abh 144:254–267
McConnell JDC, McCammon CA, Angel RJ, Seifert F (2000) The nature of the incommensurate structure in åkermanite, Ca2MgSi2O7, and the character of its transformation from the normal structure. Z Kristallogr 215:669–677
Merlini M, Gemmi M, Artioli G (2005) Thermal expansion and phase transitions in åkermanite and gehlenite. Phys Chem Miner 32:289–296
Merlini M, Gemmi M, Cruciani G, Artioli G (2008) High-temperature behavior of melilite: in situ X-ray diffraction study of gehlenite-åkermanite-Na melilite solid solution. Phys Chem Miner 35:147–155
Merlini M, Gemmi M, Hanfland M, Crichton W (2009) High-pressure behavior of åkermanite and gehlenite and phase stability of the normal structure in melilites. Am Mineralogist 94:704–709
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic elongation: a quantitative measure of distortion in coordination polyhedra. Science 172:567–570
Seifert F (1988) Recent advances in the mineralogical applications of the 57Fe Mössbauer effect. Physical properties and thermodynamic behavior of minerals, pp 687–703
Seifert F, Röthlisberger F (1993) Macroscopic and structural changes at the incommensurate-normal phase transition in melilites. Miner Petrol 48:179–192
Seifert F, Czank M, Simons B, Schmahl W (1987) A commensurate-incommensurate phase transition in iron-bearing Åkermanites. Phys Chem Miner 14:26–35
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A32:751–767
Warren BE (1930) The structure of melilite (Ca,Na)2(Mg,Al)1(Si,Al)2O7. Z Kristallogr 74:131–138
Yang H, Hazen RM, Downs RT, Finger LW (1997) Structural change associated with the incommensurate-normal phase transition in åkermanite, Ca2MgSi2O7, at high pressure. Phys Chem Miner 24:510–519
Young RA (1993) Introduction to the Rietveld method. In: Young RA (ed) The Rietveld Method. Oxford Science Publications, Oxford, pp 1–38
Acknowledgments
We thank Prof. Takuya Ohba of Department of Material Science, Shimane University, and Dr. Taisuke Hayashi of Department of Materials Analysis, CIRS, Shimane University, for their support to use RIGAKU SmartLab X-ray powder diffractometer, Drs. Fujio Izumi of the National Institute for Materials Science and Koichi Momma of the National Museum of Nature and Science for their permission to use RIETAN-FP and VESTA programs, and Dr. Barry P. Roser of Department of Geoscience, Shimane University, for his critical reading of the manuscript. We also thank the cooperation of the Center for Integrated Research in Science for providing the XRD (RIGAKU SmartLab), experimental facility, which was introduced through the Tatara Project supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan. We appreciate Dr. Masanori Matsui, the handling editor, and two anonymous reviewers for their helpful comments and advice. This work was supported by Grant-in-Aid for Scientific Research 22-1791.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamada, M., Akasaka, M. Distribution of cations at two tetrahedral sites in Ca2MgSi2O7-Ca2Fe3+AlSiO7 series synthetic melilite and its relation to incommensurate structure. Phys Chem Minerals 40, 259–270 (2013). https://doi.org/10.1007/s00269-013-0566-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-013-0566-8