Abstract
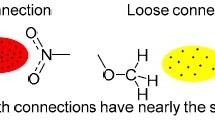
Electron density distributions, bond paths, Laplacian and local-energy density properties have been calculated for a number of As4S n (n = 3, 4 and 5) thioarsenide molecular crystals. On the basis of the distributions, the intramolecular As–S and As–As interactions classify as shared bonded interactions, and the intermolecular As–S, As–As and S–S interactions classify as closed-shell van der Waals (vdW) bonded interactions. The bulk of the intermolecular As–S bond paths link regions of locally concentrated electron density (Lewis-base regions) with aligned regions of locally depleted electron density (Lewis-acid regions) on adjacent molecules. The paths are comparable with intermolecular paths reported for several other molecular crystals that link aligned Lewis base and acid regions in a key–lock fashion, interactions that classified as long-range Lewis acid–base-directed vdW interactions. As the bulk of the intermolecular As–S bond paths (~70%) link Lewis acid–base regions on adjacent molecules, it appears that molecules adopt an arrangement that maximizes the number of As–S Lewis acid–base intermolecular bonded interactions. The maximization of the number of Lewis acid–base interactions appears to be connected with the close-packed array adopted by molecules: distorted cubic close-packed arrays are adopted for alacránite, pararealgar, uzonite, realgar and β-AsS and the distorted hexagonal close-packed arrays adopted by α- and β-dimorphite. A growth mechanism is proposed for thioarsenide molecular crystals from aqueous species that maximizes the number of long-range Lewis acid–base vdW As–S bonded interactions with the resulting directed bond paths structuralizing the molecules as a molecular crystal.


















Similar content being viewed by others
References
Bader RFW (1990) Atoms in molecules. Oxford Science Publications, Oxford
Bader RFW (1998) A bond path: a universal indicator of bonded interactions. J Phys Chem A 102(37):7314–7323
Bader RFW (2009) Bond paths are not chemical bonds. J Phys Chem A 113(38):10391–10396
Bader RFW (2010) Definition of molecular structure: by choice or by appeal to observation? J Phys Chem A 114(28):7431–7444
Bader RFW, Essén H (1984) The characterization of atomic interactions. J Chem Phys 80(5):1943–1960
Bader RFW, MacDougall PJ (1985) Toward a theory of chemical reactivity based on the charge density. J Am Chem Soc 107(24):6788–6795
Bader RFW, MacDougall PJ, Lau CDH (1984) Bonded and nonbonded charge concentrations and their relation to molecular geometry and reactivity. J Am Chem Soc 106(6):1594–1605
Bader RFW, Gillespie RJ, Macdougall PJ (1988) A physical basis for the VSEPR model of molecular-geometry. J Am Chem Soc 110(22):7329–7336
Bader RFW, Hernandez-Trujillo J, Cortes-Guzman F (2007) Chemical bonding: from Lewis to atoms in molecules. J Comput Chem 28(1):4–14
Ballirano P, Maras A (2006) In situ X-ray transmission powder diffraction study of the kinetics of the light induced alteration of realgar (alpha-As4S4). Eur J Mineral 18(5):589–599
Bindi L, Popova V, Bonazzi P (2003) Uzonite, As4S5, from the type locality: single-crystal X-ray study and effects of exposure to light. Can Mineral 41:1463–1468
Boese R, Boese AD, Blaser D, Antipin MY, Ellern A, Seppelt K (1997) The surprising crystal packing of chlorinefluoride. Angew Chem Int Ed Engl 36(13–14):1489–1492
Bonazzi P, Bindi L (2008) A crystallographic review of arsenic sulfides: effects of chemical variations and changes induced by exposure to light. Z Kristallogr 223(1–2):132–147
Bonazzi P, Menchetti S, Pratesi G (1995) The crystal-structure of pararealgar, As4S4. Am Mineral 80(3–4):400–403
Bonazzi P, Bindi L, Popova V, Pratesi G, Menchetti S (2003) Alacranite, As(8)S(9): structural study of the holotype and re-assignment of the original chemical formula. Am Mineral 88(11–12):1796–1800
Bone RGA, Bader RFW (1996) Identifying and analyzing intermolecular bonding interactions in van der Waals molecules. J Phys Chem 100(26):10892–10911
Bozorth RM (1923) The crystal structures of the cubic forms of arsenious and antimonous oxides. J Am Chem Soc 45(7):1621–1627
Bullen HA, Dorko MJ, Oman JK, Garrett SJ (2003) Valence and core-level binding energy shifts in realgar (As4S4) and pararealgar (As4S4) arsenic sulfides. Surf Sci 531(3):319–328
Burns PC, Percival JB (2001) Alacranite, As4S4: a new occurrence, new formula, and determination of the crystal structure. Can Mineral 39:809–818
Carroll MT, Bader RFW (1988) An analysis of the hydrogen-bond in BASE-HF complexes using the theory of atoms in molecules. Mol Phys 65(3):695–722
Cremer D, Kraka E (1984) A description of the chemical bond in terms of local properties of electron density and energy. Croat Chem Acta 57(6):1259–1281
Dovbeshko GI, Fesenko OM, Obraztsova ED, Allakhverdiev KR, Kaja AE (2009) Conformation analysis of nucleic acids and proteins adsorbed on single-shell carbon nanotubes. J Struct Chem 50(5):954–961
Downs RT (2000) Analysis of harmonic displacement factors. In: Hazen RM, Downs RT (eds) High-temperature and high-pressure crystal chemistry, vol 41. Mineralogical Society of America, Washington, DC
Dunitz JD, Gavezzotti A (2005) Molecular recognition in organic crystals: directed intermolecular bonds or nonlocalized bonding? Angew Chem Int Ed Engl 44(12):1766–1787
Dunitz JD, Gavezzotti A (2009) How molecules stick together in organic crystals: weak intermolecular interactions. Chem Soc Rev 38(9):2622–2633
Feldman K, Fritz M, Hahner G, Marti A, Spencer ND (1998) Surface forces, surface chemistry and tribology. Tribol Int 31(1–3):99–105
Feynman RP (1939) Forces in molecules. Phys Rev 56(4):340
Fiedler S, Broecker J, Keller S (2010) Protein folding in membranes. Cell Mol Life Sci 67(11):1779–1798
French RH, Parsegian VA, Podgornik R, Rajter RF, Jagota A, Luo J, Asthagiri D, Chaudhury MK, Chiang YM, Granick S, Kalinin S, Kardar M, Kjellander R, Langreth DC, Lewis J, Lustig S, Wesolowski D, Wettlaufer JS, Ching WY, Finnis M, Houlihan F, von Lilienfeld OA, van Oss CJ, Zemb T (2010) Long range interactions in nanoscale science. Rev Mod Phys 82(2):1887–1944
Gatti C (1997) TOPOND96 user’s manual. CNR-CSRSRC, Milan
Gibbs GV, Wallace AF, Cox DF, Dove PM, Downs RT, Ross NL, Rosso KM (2009) Role of directed van der Waals bonded interactions in the determination of the structures of molecular arsenate solids. J Phys Chem A 113(4):736–749
Gibbs GV, Wallace AF, Zallen R, Downs RT, Ross NL, Cox DF, Rosso KM (2010) Bond paths and van der Waals interactions in orpiment, As2S3. J Phys Chem A 114(23):6550–6557
Gotzinger M, Peukert W (2003) Dispersive forces of particle-surface interactions: direct AFM measurements and modelling. Powder Technol 130(1–3):102–109
Hamilton WC, Lassier B, Kay MI (1974) Phonon spectra of trigonal selenium at 77 and 298 K. J Phys Chem Solids 35(9):1089–1094
Helz GR, Tossell JA (2008) Thermodynamic model for arsenic speciation in sulfidic waters: a novel use of ab initio computations. Geochim Cosmochim Acta 72(18):4457–4468
Hillier IH, Rice SA (1967) Comments on influence of intermediate excitons and exchange forces on interaction in molecular crystals—crystal structure of chlorine. J Chem Phys 46(10):3881–3889
Ito T, Morimoto N, Sadanaga R (1952) The crystal structure of realgar. Acta Crystallogr A 5(6):775–782
Kitaigorodskii AI (1955) Organic chemical crystallography. Academy of Sciences of USSR, Moscow
Kitaigorodskii AI (1973) Molecular crystals and molecules. Academic Press, New York
Kyono A (2009) Molecular conformation and anion configuration variations for As4S4 and As4Se4 in an anion-substituted solid solution. Am Mineral 94(4):451–460
Kyono A, Kimata M, Hatta T (2005) Light-induced degradation dynamics in realgar: in situ structural investigation using single-crystal X-ray diffraction study and X-ray photoelectron spectroscopy. Am Mineral 90(10):1563–1570
Lauria A, Ippolito M, Almerico AM (2009) Inside the Hsp90 inhibitors binding mode through induced fit docking. J Mol Graph Model 27(6):712–722
Lichanot A, Apra E, Dovesi R (1993) Quantum-mechanical Hartree-Fock study of the elastic properties of Li2S and Na2S. Physica Status Solidi B Basic Res 177(1):157–163
Maitland GC, Rigby M, Smith EB, Wakeham WA (1980) Intermolecular forces: their origin and determination. Oxford University Press, Oxford, 450 pp
Makovicky E (2006) Crystal structures of sulfides and other chalcogenides. Rev Mineral Geochem 61(1):7–125
Makovicky E, Mumme WG (1983) The crystal-structure of ramdohrite, Pb6Sb11Ag3S24, and its implications for the andorite group and zinckenite. Neues Jahrb Mineral Abh 147(1):58–79
Makovicky E, Balic-Zunic T, Karanovic L, Poleti D (2006a) The crystal structure of kirkiite, Pb10Bi3As3S19. Can Mineral 44:177–188
Makovicky E, Topa D, Mumme WG (2006b) The crystal structure of dadsonite. Can Mineral 44:1499–1512
Martin RM, Lucovsky G, Helliwell K (1976) Intermolecular bonding and lattice-dynamics of Se and Te. Phys Rev B 13(4):1383–1395
Mikami B, Degano M, Hehre EJ, Sacchettini JC (1994) Crystal-structures of soybean beta-amylase reacted with beta-maltose and maltal—active-site components and their apparent roles in catalysts. Biochemistry 33(25):7779–7787
Moëlo Y, Makovicky E, Mozgova NN, Jambor JL, Cook N, Pring A, Paar W, Nickel EH, Graeser S, Karup-Moller S, Balic-Zunic T, Mumme WG, Vurro F, Topa D, Bindi L, Bente K, Shimizu M (2008) Sulfosalt systematics: a review Report of the sulfosalt sub-committee of the IMA Commission on Ore Mineralogy. Eur J Mineral 20(1):7–46
Naumov P, Makreski P, Jovanovski G (2007) Direct atomic scale observation of linkage isomerization of As4S4 clusters during the photoinduced transition of realgar to pararealgar. Inorg Chem 46(25):10624–10631
Nyburg SC (1964) Intermolecular Forces + Space Group of Solid Chlorine. J Chem Phys 40(9):2493–2501
O’Day P (2006) Chemistry and mineralogy of arsenic. Elements 2(2):77–83
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, Oxford, p 333
Pine SH, Hendrickson JB (1980) Organic chemistry. McGraw-Hill, New York
Saunders VR, Dovesi R, Roetti C, Causa M, Harrison NM, Orlando R, Apra E (1998) CRYSTAL98 user’s manual. University of Torino, Torino
Scherer W, Spiegler M, Pedersen B, Tafipolsky M, Hieringer W, Reinhard B, Downs AJ, McGrady GS (2000) Molecular recognition in the solid state: topology of experimental and theoretical charge densities for tetrasulfur tetranitride. Chem Commun (7):635–636
Slater JC (1972) Hellmann-Feynman and virial theorems in the X alpha method. J Chem Phys 57(6):2389–2396
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17(5):517–568
Sponer J, Riley KE, Hobza P (2008) Nature and magnitude of aromatic stacking of nucleic acid bases. Phys Chem Chem Phys 10(19):2595–2610
Spycher NF, Reed MH (1989) As(III) and Sb(III) sulfide complexes—an evaluation of the stoichiometry and stability from existing experimental data. Geochim Cosmochim Acta 53(9):2185–2194
Thonhauser T, Cooper VR, Li S, Puzder A, Hyldgaard P, Langreth DC (2007) Van der Waals density functional: self-consistent potential and the nature of the van der Waals bond. Phys Rev B 76(12):125112-1–125112-11
Towler MD, Dovesi R, Saunders VR (1995) Magnetic-interactions and the cooperative Jahn-Teller effect in KCuF3. Phys Rev B 52(14):10150–10159
Tsirelson VG, Zou PF, Tang TH, Bader RFW (1995) Topological definition of crystal-structure—determination of the bonded interactions in solid molecular chlorine. Acta Crystallogr A 51:143–153
Tsirelson VG, Shishkina AV, Stash AI, Parsons S (2009) The experimental and theoretical QTAIMC study of the atomic and molecular interactions in dinitrogen tetroxide. Acta Crystallogr B 65(5):647–658
Wei A, Tripp SL, Liu J, Kasama T, Dunin-Borkowski RE (2009) Calixarene-stabilised cobalt nanoparticle rings: self-assembly and collective magnetic properties. Supramol Chem 21(3–4):189–195
Welch AH, Westjohn DB, Helsel DR, Wanty RB (2000) Arsenic in ground water of the United States: occurrence and geochemistry. Ground Water 38(4):589–604
Whitfield HJ (1970) Crystal structure of tetra-arsenic trisulphide. J Chem Soc (10):1800–1803
Whitfield HJ (1973a) Crystal and molecular-structure of tetra-arsenic pentasulfide. J Chem Soc Dalton Trans (17):1740–1742
Whitfield HJ (1973b) Crystal-structure of beta-form of tetra-arsenic trisulfide. J Chem Soc Dalton Trans (17):1737–1738
Whitten AE, Dittrich B, Spackman MA, Turner P, Brown TC (2004) Charge density analysis of two polymorphs of antimony(III) oxide. Dalton Trans (1):23–29
Williams DE, Hsu LY (1985) Transferability of nonbonded Cl=Cl potential-energy function to crystalline chlorine. Acta Crystallogr A 41:296–301
Xu J, Stevens MJ, Oleson TA, Last JA, Sahai N (2009) Role of oxide surface chemistry and phospholipid phase on adsorption and self-assembly: isotherms and atomic force microscopy. J Phys Chem C 113(6):2187–2196
Zallen R, Slade M (1974) Rigid-layer modes in chacogenide crystals. Phys Rev B 9(4):1627–1637
Zallen R, Slade ML, Ward AT (1971) Lattice vibrations and interlayer interactions in crystalline As2S3 and As2Se3. Phys Rev B 3(12):4257–4273
Acknowledgments
The National Science Foundation and the US Department of Energy are thanked for supporting this study with Grants EAR-0609885 (N.L.R. and G.V.G.), EAR-0609906 (R.T.D.), and DE-FG02-97ER14751 (D.F.C.). K.M.R. acknowledges a grant from the US Department of Energy (DOE), Office of Basic Energy Sciences, Geoscience Division and computational facilities and support from the Environmental Molecular Sciences Laboratory (EMSL) at the Pacific Northwest National Laboratory (PNNL). The computations were performed in part at the EMSL at PNNL. The EMSL is a national scientific user facility sponsored by the US DOE Office of Biological and Environmental Research. PNNL is operated by Battelle for the DOE under contract DEAC06-76RLO 1830. GVG wishes to thank his good friend and colleague Professor Michael Hochella for reading a preliminary draft of the manuscript and contributing to the discussion of the growth mechanism for a thioarsenide molecular crystal that maximizes the number of long-range Lewis acid–base vdW As–S bonded interactions. He also wishes to thank Professors Richard F. W. Bader at McMaster University, Ontario, Canada and Vladimir Tsirelson at Mendelev University of Chemical Technology, Moscow, Russia for useful discussions related to van der Waals bonded interactions. We also want to thank Professor Emil Makovicky at University of Copenhagen, Copenhagen, Denmark for his careful review of the manuscript, his suggested changes and his insightful comments on the connection between micelles and directed bond paths.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gibbs, G.V., Wallace, A.F., Downs, R.T. et al. Thioarsenides: a case for long-range Lewis acid–base-directed van der Waals interactions. Phys Chem Minerals 38, 267–291 (2011). https://doi.org/10.1007/s00269-010-0402-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-010-0402-3