Abstract
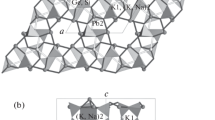
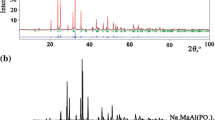
The crystallographic structures of the synthetic cheralite, CaTh(PO4)2, and its homolog CaNp(PO4)2 have been investigated by X-ray diffraction at room temperature. Rietveld analyses showed that both compounds crystallize in the monoclinic system and are isostructural to monazite LnPO4 (Ln = La to Gd). The space group is P21/n (I.T. = 14) with Z = 2. The refined lattice parameters of CaTh(PO4)2 are a = 6.7085(8) Å, b = 6.9160(6) Å, c = 6.4152(6) Å, and β = 103.71(1)° with best fit parameters R wp = 4.87%, R p = 3.69% and R B = 3.99%. For CaNp(PO4)2, we obtained a = 6.6509(5) Å, b = 6.8390(3) Å, c = 6.3537(8) Å, and β = 104.12(6)° and R wp = 6.74%, R p = 5.23%, and R B = 6.05%. The results indicate significant distortions of bond length and angles of the PO4 tetrahedra in CaTh(PO4)2 and to a lesser extent in CaNp(PO4)2. The structural distortions were confirmed by Raman spectroscopy of CaTh(PO4)2. A comparison with the isostructural compounds LnPO4 (Ln = Ce and Sm) confirmed that the substitution of the large rare earth trivalent cations with Ca2+ and Th4+ introduces a distortion of the PO4 tetrahedra.




Similar content being viewed by others
References
Aldred AT (1984) Cell volumes of APO4, AVO4 and ANbO4 compounds, where A = Sc, Y, La- Lu. Acta Crystallogr B 40:569–574. doi:10.1107/S0108768184002718
Beall GW, Boatner LA, Mullica DF, Milligan WO (1981) The structure of cerium orthophosphate, a synthetic analogue of monazite. J Inorg Nucl Chem 43:101–105. doi:10.1016/0022-1902(81)80443-5
Begun GM, Beall GW, Boatner LA, Gregor WJ (1981) Raman spectra of the rare earth orthophosphates. J Raman Spectrosc 11:248–253. doi:10.1002/jrs.1250110411
Bregiroux D, Terra O, Audubert F, Dacheux N, Serin V, Podor R et al (2007) Solid-state synthesis of monazite-type compounds containing tetravalent elements. Inorg Chem 46:10327–10382. doi:10.1021/ic7012123
Burakov BE, Yagovkina MA, Zamoryanskaya MV, Garbuzov VM, Zirlin VA, Kitsay AA (2005): Self irradiation of ceramics and single crystals doped with plutonium 238. Recent advances in actinide science, Proceedings Actinides 2005 Conference, Manchester July 2005, RSC Publishing
Dusausoy Y, Ghermani NE, Podor R, Cuney M (1996) Low-temperature ordered phase of CaU(PO4)2: synthesis and crystal structure. Eur J Mineral 8:667–673
Hikichi Y, Hukuo K, Shiokawa J (1978) Solid solutions in the systems monazite (CePO4)—huttonite (ThSiO4) and monazite—Ca0.5Th0.5PO4. Nippon Kagaku Kaishi 12:1635–1640
Jardin R, Pavel CC, Raison PE, Bouëxière D, Santa-Cruz H, Konings RJM, et al (2008) The high temperature behaviour of PuPO4 monazite and of some other related compounds. J Nucl Mater doi:10.1016/j.jnucmat.2008.05.011
Linthout K (2007) Tripartite division of the system 2REEPO4 - CaTh(PO4)2–2ThSiO4, discreditation of Brabantite, and recognition of cheralite as the name for members dominated by CaTh(PO4)2. Can Mineral 45:503–508. doi:10.2113/gscanmin.45.3.503
McCarthy GJ, White WB, Pfoertsch DE (1978) Synthesis of nuclear waste monazites, ideal actinide hosts for geologic disposal. Mater Res Bull 13:1239–1245. doi:10.1016/0025-5408(78)90215-5
Meldrum A, Boatner LA, Weber WJ, Ewing RC (1998) Radiation damage in zircon and monazite. Geochim Cosmochim Acta 62:2509–2520. doi:10.1016/S0016-7037(98)00174-4
Montel JM, Devidal JL, Avignant D (2002) X-ray diffraction study of brabantite-monazite solid solutions. Chem Geol 191:89–104. doi:10.1016/S0009-2541(02)00150-X
Mullica DF, Grossie DA, Boatner LA (1985) Coordination geometry and structural determinations of SmPO4, EuPO4 and GdPO4. Inorg Chim Acta 109:105–110. doi:10.1016/S0020-1693(00)84549-1
Ni Y, Hughes JM, Mariano AN (1995) Crystal chemistry of the monazite and xenotime structures. Am Mineral 80:21–26
Popović L, de Waal D, Boeyens JCA (2005) Correlation between Raman wavenumbers and P–O bond lengths in crystalline inorganic phosphates. J Raman Spectrosc 36:2–11. doi:10.1002/jrs.1253
Podor R, Cuney M, Nguywn Trung C (1995) Experimental study of the solid solution between monazite-(La) and (Ca0.5U0.5)PO4 at 780°C and 200 MPa. Am Mineral 80:1261–1268
Podor R (1995) Raman spectra of the actinide-bearing monazites. Eur J Mineral 7:1353–1360
Podor R, Cuney M (1997) Experimental study of Th-bearing (780°C, 200 MPa); implication for monazite and actinide orthophosphate stability. Am Mineral 82:765–771
Popa, K, Shvareva, T., Mazeina, L., Colineau, E., Wastin, F., Konings, R.J.M., Navrotsky, A. (2008) Thermodynamic properties of CaTh(PO4)2 synthetic cheralite. Am Mineral 93(8–9)
Rodriguez-Carjaval J (1993) Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 192:55–69. doi:10.1016/0921-4526(93)90108-I
Rose D (1980) Brabantite, CaTh[PO4]2, a new mineral of the monazite group. N Jb Min Mh 6:247–257
Seydoux-Guillaume AM, Wirth R, Nasdala L, Gottschalk M, Montel JM, Heinrich W (2002) An XRD, TEM and Raman study of experimentally annealed natural monazite. Phys Chem Miner 29:59–62. doi:10.1007/s00269-001-0232-4
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalshogenides. Acta Crystallogr A 32:751–766. doi:10.1107/S0567739476001551
Silva EN, Ayala AP, Guedes I, Paschoalm CWA, Moreiram RL, Loong CK et al (2002) Vibrational spectra of monazite-type rare-earth orthophosphates. Opt Mater 29:224–230. doi:10.1016/j.optmat.2005.09.001
Tabuteau A, Pagès M, Livet J, Musikas C (1986) Crystallochemical properties of transuranium phosphates. J Less Common Met 121:650–651. doi:10.1016/0022-5088(86)90590-4
Tabuteau A, Pagès M, Livet J, Musikas C (1988) Monazite-like phases containing transuranium elements (neptunium and plutonium). J Mater Sci Lett 7:1315–1317. doi:10.1007/BF00719969
Acknowledgments
We thank Dr. G. Novitchi (State University, Chisinau, Moldova) for his assistance during the IR measurements. K.P. and C.C.P. acknowledges the European Commission for support given in the frame of program “Training and Mobility of Researchers”. Participation to the European Commission-JRC-ITU Actinide User Laboratory program is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raison, P.E., Jardin, R., Bouëxière, D. et al. Structural investigation of the synthetic CaAn(PO4)2 (An = Th and Np) cheralite-like phosphates. Phys Chem Minerals 35, 603–609 (2008). https://doi.org/10.1007/s00269-008-0252-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-008-0252-4