Abstract
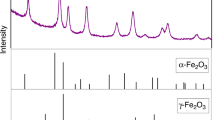
A multi-anvil device was used to synthesize 24 mg of pure γ-Fe2SiO4 crystals at 8.5 GPa and 1,273 K. The low-temperature heat capacity (C p) of γ-Fe2SiO4 was measured between 5 and 303 K using the heat capacity option of a physical properties measurement system. The measured heat capacity data show a broad λ-transition at 11.8 K. The difference in the C p between fayalite and γ-Fe2SiO4 is reduced as the temperature increases in the range of 50–300 K. The gap in C p data between 300 and 350 K of γ-Fe2SiO4 is an impediment to calculation of a precise C p equation above 298 K that can be used for phase equilibrium calculations at high temperatures and high pressures. The C p and entropy of γ-Fe2SiO4 at standard temperature and pressure (S°298) are 131.1 ± 0.6 and 140.2 ± 0.4 J mol−1 K−1, respectively. The Gibbs free energy at standard pressure and temperature (ΔG° f,298) is calculated to be −1,369.3 ± 2.7 J mol−1 based on the new entropy data. The phase boundary for the fayalite–γ-Fe2SiO4 transition at 298 K based on current thermodynamic data is located at 2.4 ± 0.6 GPa with a slope of 25.4 bars/K, consistent with extrapolated results of previous experimental studies.



Similar content being viewed by others
References
Akaogi M, Ito E, Navrotsky A (1989) Olivine-modified spinel–spinel transition in the system Mg2SiO4–Fe2SiO4: calorimetric measurements, thermochemical calculation, and geophysical application. J Geophys Res 94:15671–15685
Akimoto S, Fujisawa H, Katsura T (1965) Olivine–spinel transition in Fe2SiO4 and Ni2SiO4. J Geophys Res 66:1969–1977
Akimoto S, Komada E, Kushiro I (1967) Effect of pressure on the melting of olivine and spinel polymorphs of Fe2SiO4. J Geophys Res 68:679–686
Akimoto S, Matsui Y, Syono Y (1976) High-pressure crystal chemistry of orthosilicates and formation of the mantle transition zone. In: Strens RGJ (eds) The physics and chemistry of minerals and rocks. Wiley, New York, pp 327–363
Akimoto S, Yagi T, Inoue K (1977) High temperature–pressure phase boundaries in silicate systems using in situ X-ray diffraction. In: Manghnani MH, Akimoto S (eds) High-pressure research: application in geophysics. Academic, New York, pp 585–602
Dachs E, Bertoldi C (2005) Precision and accuracy of the heat-pulse calorimetric technique: low temperature heat capacities of milligram-sized synthetic mineral samples. Eur J Mineral 17:251–259
Dachs E, Geiger CA (2006) Heat capacities and entropies of mixing of pyrope–grossular (Mg3Al2Si3O12–Ca3Al2Si3O12) garnet solid solutions: a low-temperature calorimetric and a thermodynamic investigation. Am Mineral 91:8894–9006
Ehrenberg H, Fuess H (1993) Analytical interpretation and simulation of the static magnetic properties of synthetic α-Fe2SiO4. J Phys Condens Matter 5:3663–3672
Fabrichnaya O (1998) The assessment of thermodynamic parameters for solid phases in the Fe–Mg–O and Fe–Mg–Si–O systems. Calphad 22:85–125
Fei Y, Saxena SK (1986) A thermochemical data base for phase equilibria in the system Fe–Mg–Si–O at high pressure and temperature. Phys Chem Miner 13:311–324
Fei Y, Mao H, Mysen BO (1991) Experimental determination of element partitioning and calculation of phase relations in the MgO–FeO–SiO2 system at high pressure and high temperature. J Geophys Res 96:2157–2169
Furnish MD, Bassett WA (1983) Investigation of the mechanism of the olivine–spinel transition in fayalite by synchrotron radiation. J Geophys Res 88:10333–10341
Gopal ESR (1966) Specific heats at low temperatures. Plenum, New York
Inoue K (1975) Development of high temperature and high pressure X-ray diffraction apparatus with energy dispersive technique and its geophysical applications. Ph.D. thesis, Tokyo University, Tokyo
Jacobs MHG, de Jong BHWS (2005) An investigation into thermodynamic consistency of data for the olivine, wadsleyite and ringwoodite form of (Mg,Fe)2SiO4. Geochim Cosmochim Acta 69:4361–4375
Jacobs MHG, de Jong BHWS, Oonk HAJ (2001) The Gibbs energy formulation of α, γ, and liquid Fe2SiO4 using Grover, Getting and Kennedy’s empirical relation between volume and bulk modulus. Geochim Cosmochim Acta 65:4231–4242
Kudoh Y, Takeda H (1986) Single crystal X-ray diffraction study on the bond compressibility of fayalite, Fe2SiO4 and rutile, TiO2 under high pressure. Physica B 139–140:333–336
Lashley JC, Hundley MF, Migliori A, Sarrao JL, Pagliuso PG, Darling TW, Jaime M, Cooley JC, Hults WL, Morales L, Thoma DJ, Smith JL, Boerio-Goates J, Woodfield BF, Stewart GR, Fisher RA, Phillips NE (2003) Critical examination of heat capacity measurements made on a quantum design physical property measurement system. Cryogenics 43:369–378
Marumo F, Isobe M, Akimoto S (1977) Electron-density distributions in crystals of γ-Fe2SiO4 and γ-Co2SiO4. Acta Crystallogr B 33:713–716
Robie RA, Hemingway BS (1972) Calorimeters for heat of solution and low-temperature heat capacity measurement. U.S. Geol Surv Prof Paper 755, 32 pp
Robie RA, Hemingway BS (1995) Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures. US Geol Surv Bull 2131:461
Robie RA, Finch CB, Hemingway BS (1982) Heat capacity and entropy of fayalite (Fe2SiO4) between 5.1 and 383 K: comparison of calorimetric and equilibrium values of the QFM buffer reaction. Am Mineral 67:463–469
Santoro RP, Newnham RE, Nomura S (1966) Magnetic properties of Mn2SiO4 and Fe2SiO4. J Phys Chem Solids 27:655–666
Sato Y (1977) Equation of state of mantle minerals determined through high-pressure X-ray study. In: Manghnani MH, Akimoto S (eds) High-pressure research: application in geophysics. Academic, New York, pp 307–323
Sung CM, Burns RG (1976) Kinetics of high-pressure phase transformations: implications to the evolution of the olivine–spinel transition in the downgoing lithosphere and its consequences on the dynamics of the mantle. Tectonophysics 31:1–32
Watanabe H (1982) Thermochemical properties of synthetic high-pressure compounds relevant to the earth’s mantle. In: Manghnani MH, Akimoto S (eds) High-pressure research in geophysics. Center for Academic Publications, Japan, pp 441–464
Xie ZD, Sharp TG (2004) High-pressure phases in shock-induced melt veins of the Umbarger L6 chondrite: constraints of shock pressure. Meteorit Planet Sci 39:2043–2054
Xie ZD, Tomioka N, Sharp TG (2002) Natural occurrence of Fe2SiO4–spinel in the shocked Umbarger L6 chondrite. Am Mineral 87:1257–1260
Yagi T, Akaogi M, Shimomura O, Suzuki T, Akimoto S (1987) In situ observation of the olivine–spinel phase transition in Fe2SiO4 using synchrotron radiation. J Geophys Res 92:6207–6213
Yong W, Dachs E, Withers AC, Essene EJ (2006) Heat capacity and phase equilibria of hollandite polymorph of KAlSi3O8. Phys Chem Miner 33:167–177
Acknowledgments
The authors are grateful to C. Henderson for his help in EMP analysis, and R.C. Rouse for his help with XRD measurements. We gratefully acknowledge the constructive reviews of K.-D. Grevel and one anonymous reviewer, which improved the quality of the manuscript. This work was supported by Scott Turner Research Grant by the Department of Geological Sciences, University of Michigan to the senior author, NSF grants EAR 96-28196, 99-11352, 00-87448 and 05-37068 to E.J. Essene, NSF grants EAR 03-10142 and 00-79827 to M. Hirschmann at University of Minnesota, and grant P15880-N11 of the Austrian Science Fund to E. Dachs at the University of Salzburg.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yong, W., Dachs, E., Withers, A.C. et al. Heat capacity of γ-Fe2SiO4 between 5 and 303 K and derived thermodynamic properties. Phys Chem Minerals 34, 121–127 (2007). https://doi.org/10.1007/s00269-006-0133-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0133-7