Abstract
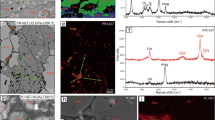
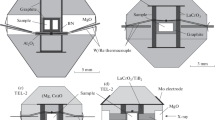
High-pressure and temperature experiments (28–62 GPa, and 1,490–2,000 K, corresponding to approximately 770–1,500 km depth in the mantle) have been conducted on a MgCO3 + SiO2 mixture using a laser-heated diamond anvil cell combined with analytical transmission electron microscope observation of the product phases to constrain the fate of carbonates carried on the subducting basalt into the lower mantle. At these conditions, the decarbonation reaction MgCO3 (magnesite) + SiO2 (stishovite) → MgSiO3 (perovskite) + CO2 (solid) has been recognized. This indicates that above reaction takes place as a candidate for decarbonation of the carbonated subducting mid ocean ridge basalts in the Earth’s lower mantle.



Similar content being viewed by others
References
Akaogi M, Ito E, Navrotsky A (1989) Olivine-modified spinel-spinel transitions in the system Mg2SiO4-Fe2SiO4: calorimetric measurements, thermochemical calculation, and geophysical application. J Geophys Res 94:15671–15685
Andrault D, Angel RJ, Mosenfelder JL, Le Bihan T (2000) Equation of state of stishovite to lower mantle pressures. Am Mineral 88:301–307
Biellmann C, Gillet P, Guyot F, Peyronneau J, Reynard B (1993) Experimental evidence for carbonate stability in the Earth’s lower mantle. Earth Planet Sci Lett 118:31–41
Brown JM, Shankland TJ (1981) Thermodynamic parameters in the Earth as determined from seismic profiles. Geophys J R Astron Soc 66:620–622
Canil D, Scarfe CM (1990) Phase relations in Peridotite + CO2 systems to 12 GPa: implications for the origin of Kimberlite and carbonate stability in the Earth’s upper mantle. J Geophys Res 214:357–368
Fiquet G, Guyot F, Kunz M, Matas J, Andrault D, Hanfland M (2002) Structural refinements of magnesite at very high pressure. Am Mineral 87(8–9):1261–1265
Iota V, Yoo CS, Cynn H (1999) Quartzlike carbon dioxide: an optically nonlinear extended solid at high pressures and temperatures. Science 283:1510–1513
Iota V, Yoo CS (2001) Phase diagram of carbon dioxide: evidence for a new associated phase. Phys Rev Lett 86:5922–5925
Isshiki M, Irifune T, Hirose K, Ono S, Ohishi Y, Watanuki T, Nishibori E, Takata M, Sakata M (2004) Stability of magnesite and its high-pressure form in the lowermost mantle. Nature 427:60–63
Irifune T, Ringwood AE, Hibberson WO (1986) The eclogite-garnet transformation at high pressure and some geophysical implications. Earth Planet Sci Lett 77:245–256
Katsura T, Ito E (1990) Melting and subsolidus phase relations in the MgSiO3-MgCO3 system at high pressures: implications to evolution of the Earth’s atmosphere. Earth Planet Sci Lett 99:110–117
Knoche R, Sweeney RJ, Luth RW (1999) Carbonation and decarbonation of eclogites: the role of garnet. Contrib Mineral Petrol 135:332–339
Komabayashi T, Omori S, Maruyama S (2004) Petrogenetic grid in the system MgO-SiO2-H2O up to 30 GPa, 1600 °C: application to hydrous peridotite subducting into the Earth’s deep interior. J Geophys Res 109:B03206. DOI 10.1029/2003 JB 002651
Luth RW (1995) Experimental determination of the reaction dolomite + 2 coesite = diopside + 2 CO2 to 6 GPa. Contrib Mineral Petrol 122:152–158
Mao HK, Bell PM, Shaner J (1978) Specific volume measurements of Cu, Mo, Pd, and Ag and calibration of the ruby R1 fluorescence gauge from 0.06 to 1 Mbar. J Appl Phys 49:3276–3283
Park JH, Yoo CS, Iota V, Cynn H, Nicol MF, Le Bihan T (2003) Crystal structure of bent carbon dioxide phase IV. Phys Rev B 68:014107-1–014107-9
Santoro M, Lin JF, Mao HK, Hemley RJ (2004) In situ high P- T Raman spectroscopy and laser heating of carbon dioxide. J Che Phys 121:2780–2787
Shim SH, Duffy TS (2000) Constraints on the P-V-T equation of state of MgSiO3 perovskite. Am Mineral 85:354–363
Takafuji N, Hirose K, Ono S, Xu F, Mitome M, Bando Y (2004) Segregation of core melts by permeable flow in the lower mantle. Earth Planet Sci Lett 224:249–257
Thompson AB (1992) Water in the Earth’s mantle. Nature 358:295–302
Tschauner O, Mao HK, Hemley RJ (2001) New transformations of CO2 at high pressures and temperatures. Phys Rev Lett 87:075701-1–075701-4
Yoo CS, Cynn H, Gygi F, Galli G, Iota V, Nicol M, Carlson S, Häusermann D, Mailhiot C (1999) Crystal structure of carbon dioxide at high pressure: “Superhard” polymeric carbon dioxide. Phys Rev Lett 83:5527–5530
Yoo CS, Kohlmann H, Cynn H, Nicol MF, Iota V, LeBihan T (2002) Crystal structure of pseudo-six-fold carbon dioxide phase at high pressures and temperatures. Phys Rev B 65:104103-1–104103-6
Acknowledgments
This research was supported by a 21st Century Center of Excellence (COE) Program on “Neo-Science of Natural History” (Program Leader: Hisatake Okada) at Hokkaido University financed by the Ministry of Education, Culture, Sports, Science and Technology, Japan. Nagai was supported by Grant-in-Aid for Research (No. 18340167) from the Ministry of Education, Science, and Culture of Japan. The sample of magnesite is a specimen of the National Science Museum, Tokyo with the registration number of NSM-MF10684. We thank two anonymous reviewers for their critical and constructive comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takafuji, N., Fujino, K., Nagai, T. et al. Decarbonation reaction of magnesite in subducting slabs at the lower mantle. Phys Chem Minerals 33, 651–654 (2006). https://doi.org/10.1007/s00269-006-0119-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0119-5