Abstract
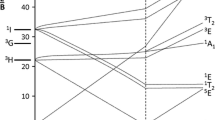
We have studied the polarized optical absorption and the EPR spectra of Ni-doped beryls grown by hydrothermal, flux and gas-transport methods, and chrysoberyl grown by the Czochralski and flux methods. In beryls, three groups of bands belonging to three various Ni centres were distinguished by analysis of the absorption band intensities. The first group, bands with maximums at 21740 (E ⊥ c), 17240 (E || c) and 9260 (E ⊥ + || c), 7140 (E || + ⊥ c) cm−1, are due to Ni3+ in octahedral Al3+ site. The second group is bands at 25640 (E ⊥ c), 22220 (E || c) and 13520 (E || + ⊥ c), 13160 (E ⊥+ || c) cm−1 and 8930 (E ⊥ + || c), 7460 (E || c) cm−1, which are caused by Ni2+ in octahedral Al3+ site. Weak wide bands at 17540 (E ⊥ c), 15500 (E || c) cm−1 and 6580 (E || + ⊥ c), 5950 (E || c) cm−1 are related to Ni2+ in tetrahedral Be2+ site. The occurrence of Ni ions in Be2+ site is proved by the EPR spectra of 1VNi+ in γ-irradiated samples. According to the spectra of optical absorption of Ni-doped chrysoberyl, two types of Ni centres have been established: Ni3+ and Ni2+ ions in octahedral Al3+ sites. From the EPR spectra of the X-ray irradiated crystals BeAl2O4: Ni, it follows that 68% of Ni+ ions occupy octahedral Al3+ sites with mirror symmetry and 32% are in Al3+ sites with inversion symmetry. In the approximation of trigonal field with regard to Trees correction, the energy levels of Ni3+ and Ni2+ have been calculated in octahedral and tetrahedral coordination. There is good agreement between the obtained experimental and calculated data. The polarization dependence of the optical absorption bands is well explained in terms of the spin–orbit interaction.






Similar content being viewed by others
References
Aines RD, Rossman GR (1984) The high-temperature behavior of water and carbon dioxide in cordierite and beryl. Am Mineral 69:319–327
Alimpiev AI, Bukin GV, Matrosov VN, Pestryakov EV, Solntsev VP, Trunov VI, Tsvetkov EG, Chebotaev VP (1986) A tunable BeAl2O4: Ti3+ laser (in Russian). Kvantovaya Electronika 14:885–886
Aurisicchio C, Fioravanti G, Grubessi O, Zanazzi PF (1988) Reappraisal of the chemistry of beryl. Am Mineral 73:826–837
Bakakin VV, Rylov GM, Belov NV (1967) Correlation between chemical composition and unit cell parameters of beryl (in Russian). Dokl Acad Sci USSR Earth Sci 173:129–132
Barry WR, Troup GI (1970) EPR of Fe3+ ions in chrysoberyl. Phys Status Sol B 38:229–234
Bukin GV, Matrosov VN, Orekhova VR, Remigailo YuL, Sevastyanov BK, Symonov EG, Solntsev VP, Tsvetkov EG (1981) Growth of alexandrite crystals and investigation of their properties. J Crystal Growth 52:537–541
Carrington A, McLachlan AD (1970) Magnetic resonance and his application in chemistry. Mir, Moscow, 447 p (translated from Carrington A, McLachlan AD (1967) Introduction to magnetic resonance with applications to chemistry and chemical physics. Harper and Row Publishers, inc, New York)
Dvir M, Low W (1960) Paramagnetic resonance and optical spectrum of iron in beryl. Phys Rev 119:1587–1591
Edgar A, Vance ER (1977) Electron paramagnetic resonance, optical absorption and magnetic circular dichroism studies of CO −3 molecular-ion in irradiated natural beryl. Phys Chem Minerals 1:165–178
Farrell EF, Fang JH, Newnham RE (1963) Refinement of the chrysoberyl structure. Am Mineral 48:804–810
Feklichev VG (1963) Chemical composition of minerals of the beryl group (in Russian). Geokhimiya 3:391–401
Forbes CE (1983) Analysis of the spin-Hamiltonian parameters for Cr3+ in mirror and inversion symmetry sites of alexandrite (Al2−x Cr x BeO4). Determination of the relative site occupancy by EPR. J Chem Phys 79:2590–2599
Hayes W, Wilkens J (1964) Proc Roy Soc. London Ser A 281:340–345
Hoffmann SK, Goslar J (1982) Crystal field theory and EPR parameters in D 2 and C 2v distorted tetrahedral copper (II) complexes. J Solid State Chem 44:343–353
Geusic IE, Peter M, Schulz-du-Bois OE (1959) Paramagnetic resonance spectrum of Cr3+ in emerald. Bell Syst Techn J 38:291–296
Ginsburg DS (1955) On the question of the composition of beryl (in Russian). Trans Min Acad Sci USSR 7:56–69
Goldman DS, Rossman GR, Parkin KM (1978) Channel constituents in beryl. Phys Chem Minerals 3:225–235
Gulev VS, Eliseev AP, Solntsev VP, Khranenko GG, Yurkin AM (1987) Flash-lamp pumped laser using emerald grown by the flux method (in Russian). Kvantovaya Electronika 14:1990–1992
Gusev VA, Eliseev AP, Samoilova EG, Solntsev VP, Yurkin AM (1988) Optical and EPR spectroscopy of chrysoberyl crystals doped with nickel (in Russian). J Appl Spectrosc 48:772–778
Khranenko GG, Solntsev VP (1988) Isomorphous substitutions in synthetic beryls (in Russian). In: Trudy Instituta Geologii i Geofiziki, Akademiya Nauk SSSR, is 487. Novosibirsk, pp 94–99
Lebedev AS, Klyakhin VA, Solntsev VP (1988) Crystal chemistry features of hydrothermal beryls (in Russian). In: Trudy Instituta Geologii i Geofiziki, Akademiya Nauk SSSR, 708. Novosibirsk, pp 75–94
Lever ABP, Hollebone BP (1972) A theoretical study of the electronic spectra of trigonally distorted transition metal complexes. I. d1, d3, d8, and d9 complexes. J Am Chem Soc 94:1816–1823
Mashkovtsev RI, Solntsev VP (2002) Channel constituents in synthetic beryl: ammonium. Phys Chem Minerals 29:65–71
Morosin B (1972) Structure and thermal expansion of beryl. Acta Cryst B 28:1899–1903
Morton JR, Preston KF (1978) Atomic parameters for paramagnetic resonance date. J Magn Res 30:577–582
Pappalardo R, Wood DL, Linares RC (1961a) Optical absorption study of Co-doped oxide system. J Chem Phys 35:2041–2059
Pappalardo R, Wood DL, Linares RC (1961b) Optical absorption study of Ni-doped oxide system. J Chem Phys 35:1460–1478
Rahman HU, Runciman WA (1971) Energy levels and g-values of vanadium in corundum. J Phys C Solid State Phys 4:1576–1590
Rodionov AYa, Solntsev VP, Weis NS (1987) Crystallization and properties of colouring varieties of gas-transport beryl (in Russian). In: Trudy Instituta Geologii i Geofiziki, Akademiya Nauk SSSR, 679 Novosibirsk, pp 41–53
Rodionov AYa, Novgorodtseva NA (1988) Crystallization of the colored varieties of chrysoberyl by the solution-melt and gas-transport methods (in Russian). In: Trudy Instituta Geologii i Geofiziki, Akademiya Nauk SSSR, 708 Novosibirsk, pp 182–187
Shand ML, Chine CF (1982) A tunable emerald laser. IEEE J Quantum Electron QE-18:1829–1830
Solntsev VP (1981) The nature of colour centers and EPR in beryl and chrysoberyl (in Russian). In: Trudy Instituta Geologii i Geofiziki, Akademiya Nauk SSSR, 499. Novosibirsk, pp 92–140
Solntsev VP, Khranenko GG (1989) The EPR of radiation defects in beryl. Sov Phys Solid State 31:292–295
Solntsev VP, Lebedev AS, Pavlyuchenko VS, Klyakhin VA (1976) Copper centers in synthetic beryl (in Russian). Sov Phys Solid State 46:1396–1398
Solntsev VP, Tsvetkov EG, Alimpiev AI, Mashkovtsev RI (2004) Valent state and coordination of cobalt ions in beryl and chrysoberyl crystals. Phys Chem Minerals 31:1–11
Sviridov DT, Sviridova RK, Smirnov YuF (1976) Optical spectra of transition metal ions in crystals (in Russian). USSR, Nauka, Moscow, 266 p
Trees RE (1951) Configuration interaction in Mn (II). Phys Rev 83:756–760
Trees RE (1952) The L(L+1) correction to the Slater formulas for the energy levels. Phys Rev 85:382
Tsvetkov EG (1982) Growth and investigation of some properties of the crysoberyl crystals (in Russian). PhD Thesis, Institute of Geology and Geophysics SB RAS USSR
Veremeichik TF, Grechushnikov BN, Kalinkina IN, Sviridov DT (1977) Trees correction for d3-configuration in a strong field scheme. Configuration of d3-electron in trigonal field (in Russian). Z Prikladnoi Spektrosk 26:131–136
Walling JC, Peterson OG, Jenssen HP, Morris RC, O′Dell EW (1980) Tunable alexandrite lasers. IEEE J Quantum Electron QE-16:1302–1314
Wood DL, Nassau K (1968) The characterization of beryl and emerald by visible and infrared absorption spectroscopy. Am Mineral 53:777–800
Acknowledgements
We thank Dr. G.G. Khranenko, Dr. A.S. Lebedev (deceased), N.A. Novgorodtseva (deceased), and Dr. A.Ya. Rodionov for the kindly donation of samples used in this study. This work was supported by the Russian Foundation for Basic Research (Grant 06-05-64395).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solntsev, V.P., Tsvetkov, E.G., Alimpiev, A.I. et al. Coordination and valent state of nickel ions in beryl and chrysoberyl crystals. Phys Chem Minerals 33, 300–313 (2006). https://doi.org/10.1007/s00269-006-0076-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0076-z