Abstract
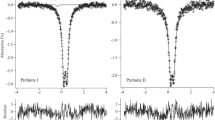
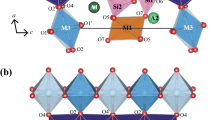
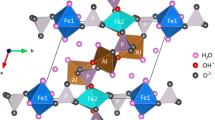
Two pumpellyites with the general formula W 8 X 4 Y 8 Z 12O56-n (OH) n were studied using 57Fe Mössbauer spectroscopic and X-ray Rietveld methods to investigate the relationship between the crystal chemical behavior of iron and structural change. The samples are ferrian pumpellyite-(Al) collected from Mitsu and Kouragahana, Shimane Peninsula, Japan. Rietveld refinements gave Fe(X):Fe(Y) ratios (%) of 41.5(4):58.5(4) for the Mitsu pumpellyite and 46(1):54(1) for the Kouragahana pumpellyite, where Fe(X) and Fe(Y) represent Fe content at the X and Y sites, respectively. The Mössbauer spectra consisted of two Fe2+ and two Fe3+ doublets for the Mitsu pumpellyite, and one Fe2+ and two Fe3+ doublets for the Kouragahana pumpellyite. In terms of the area ratios of the Mössbauer doublets and the Fe(X):Fe(Y) ratios determined by the Rietveld refinements, Fe2+(X):Fe3+(X):Fe3+(Y) ratios are determined to be 22:14:64 for the Mitsu pumpellyite and 27:8:65 for the Kouragahana pumpellyite. By applying the Fe2+:Fe3+-ratio determined by the Mössbauer analysis and the site occupancies of Fe at the X and Y sites given by the Rietveld method together with chemical analysis, the resulting formula of the Mitsu and Kouragahana pumpellyites are established as Ca8(Fe 2+0.88 Mg0.68Fe 3+0.77 Al1.66)Σ3.99(Al5.67Fe 3+2.34 )Σ8.01Si12O42.41(OH)13.59 and Ca8(Mg1.24Fe 2+0.65 Fe 3+0.46 Al1.66)Σ4.01(Al6.71Fe 3+1.29 )Σ8.00Si12O42.14(OH)13.86, respectively. Mean Y–O distances and volumes of the YO6 octahedra increase with increasing mean ionic radii, i.e., the Fe3+→Al substitution at the Y site. However, change of the sizes of XO6 octahedra against the mean ionic radii at the X site is not distinct, and tends to depend on the volume change of the YO6 octahedra. Thus, the geometrical change of the YO6 octahedra with Fe3+→Al substitution at the Y site is essential for the structural changes of pumpellyite. The expansion of the YO6 octahedra by the ionic substitution of Fe3+ for Al causes gradual change of the octahedra to more symmetrical and regular forms.








Similar content being viewed by others
References
Akasaka M (1990) Clinopyroxene on the join CaMgSi2O6–CaFe3+ AlSiO6–CaTiAl2O6 at low oxygen fugacity. Proc Indian Acad Sci (Earth Planet Sci) 99:39–48
Akasaka M, Shinno I (1992) Mössbauer spectroscopy and its recent application to silicate mineralogy (in Japanese). J Mineral Soc Japan 21:3–20
Akasaka M, Kumura Y, Omori Y, Sakakibara M, Shinno I, Togari K (1997) 57Fe Mössbauer study of pumpellyite–okhotskite–julgoldite series minerals. Mineral Petrol 61:181–198
Akasaka M, Hashimoto H, Makino K, Hino R (2003) 57Fe Mössbauer and X-ray Rietveld studies of ferrian prehnite from Kouragahana, Shimane Peninsula, Japan. J Mineral Petrol Sci 98:31–40
Allmann R, Donnay G (1973) The crystal structure of julgoldite. Miner Mag 39:271–281
Artioli G, Geiger CA (1994) The crystal chemistry of pumpellyite: an X-ray Rietveld refinement and 57Fe Mössbauer study. Phys Chem Minerals 20:443–453
Artioli G, Pavese A, Belotto M, Collins SP, Luchetti G (1996) Mn crystal chemistry in pumpellyite: a resonant-scattering powder diffraction Rietveld study using synchrotron radiation. Am Mineral 81:603–610
Artioli G, Geiger CA, Dapiaggi M (2003) The crystal chemistry of julgoldite-Fe3+ from Bombay, India, studied using synchrotron X-ray powder diffraction and 57Fe Mössbauer spectroscopy. Am Mineral 88:1084–1090
Bancroft GM, Maddock AG, Burns RG (1967) Applications of the Mössbauer effect to silicate mineralogy—I. Iron silicates of known crystal structure. Geochim Cosmochim Acta 31:2219–2246
Bancroft GM, Williams PGL, Burns RG (1971) Mössbauer spectra of minerals along the diopside-hedenbergite tie line. Am Mineral 56:1617–1625
Baur H (1974) The geometry of polyhedral distortions. Predictive relationships for the phosphate group. Acta Crystallogr B 30:1195–1215
Brese NE, O’Keeffe M (1991) Bond-valence parameters for solids. Acta Crystallogr B 47:192–197
Brown ID, Altermatt D (1985) Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta crystallogr B 41:244–247
Brown ID, Shannon RD (1973) Empirical bond-strength–bond-length curves for oxides. Acta Crystallogr A 29:266–282
Dollase WA (1986) Correction of intensities for preferred orientation in powder diffractometry: application of the March model. J Appl Crystallogr 19:267–272
Donnay G, Allmann R (1970) How to recognize O2−, OH-, and H2O in crystal structures determined by X-rays. Am Mineral 55:1003–1015
Galli E, Alberti A (1969) On the crystal structure of pumpellyite. Acta Crystallogr B 25:2276–2281
Hill RJ, Flack HD (1987) The use of the Durbin–Watson d statistic in Rietveld analysis. J Appl Crystallogr 20:356–361
Ivanov OK, Arkhangel’skaya VA, Miroshnikova LO, Shilova TA (1981) Shuiskite, the chromium analogue of pumpellyite, from the Bisersk deposit, Urals (in Russian). Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva 110:508–512
Izumi F (1993) Rietveld analysis program RIETAN and PREMOS and special applications. In: Young RA (ed) The Rietveld method. Oxford Science Publications, Oxford, pp 236–253
Izumi F (2005) VENUS system for three-dimensional visualization of crystal structures and electronic states (in Japanese with English abstract). Rigaku J 36:18–27
Izumi F, Ikeda T (2000) A Rietveld analysis program RIETAN-98 and its application to zeolites. Material Science Forum 321–324
Kano K, Satoh H, Bunno M (1986) Iron-rich pumpellyite and prehnite from the Miocene gabbroic sills of the Shimane Peninsula, Southwest Japan. J Jpn Assoc Mineral Petrol Econ Geol 81:51–58
Matsui Y, Maeda Y, Shono Y (1970) Mössbauer study of synthetic calcium-rich pyroxenes. Geochem J 14:15–26
Moore PB (1971) Julgoldite, the Fe2+–Fe3+ dominant pumpellyite. A new mineral from Långban, Sweden. Lithos 4:93–99
Nagashima M, Akasaka M (2004) An X-ray Rietveld study of piemontite on the join Ca2Al3Si3O12(OH)–Ca2Mn3+ 3Si3O12(OH) formed by hydrothermal synthesis. Am Mineral 89:1119–1129
Palache C, Vassar HE (1925) Some minerals of the Keweenawan copper deposits: a new mineral; sericite, saponite. Am Mineral 10:412–428
Passaglia E, Gottardi G (1973) Crystal chemistry and nomenclature of pumpellyites and julgoldites. Can Mineral 12:219–223
Peacor DR (1967) Refinement of the crystal structure of a pyroxene of formula MIMII (Si1.5Al0.5)O6. Am Mineral 52:31–41
Raudsepp M, Hawthorne FC, Turnock AC (1990) Evaluation of the Rietveld method for the characterization of fine-grained products of mineral synthesis: the diopside–hedenbergite join. Can Mineral 28:93–109
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic elongation: a quantitative measure of distortion in coordination polyhedra. Science 172:567–570
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32:751–767
Togari K, Akasaka M (1987) Okhotskite, a new mineral, an Mn3+-dominant member of the pumpellyite group, from the Kokuriki mine, Hakkaido, Japan. Min Mag 51:611–614
Toraya H (1990) Array-type universal profile function for powder pattern fitting. J Appl Crystallogr 23:485–491
Yoshiasa A, Matsumoto T (1985) Crystal structure refinement and crystal chemistry of pumpellyite. Am Mineral 70:1011–1019
Young RA (1993) Introduction to the Rietveld method. In: Young RA (ed) The Rietveld method. Oxford Science Publications, Oxford, pp 1–38
Acknowledgements
Our thanks to Dr. Fujio Izumi of the National Institute for his permission to use the RIETAN-2000 and VICS programs and for his helpful advice; to Dr. Kuniaki Makino of Shinshu University for helpful discussion on crystal structure analysis; and to Dr. Barry Roser of Shimane University for comment on the manuscript; Mr. Chihiro Fukuda for providing the Kouragahana pumpellyite-(Al) sample. A part of this work was supported by the Sasakawa Scientific Research Grant from the Japan Science Society to Mariko Nagashima (No. 17-105).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagashima, M., Ishida, T. & Akasaka, M. Distribution of Fe among octahedral sites and its effect on the crystal structure of pumpellyite. Phys Chem Minerals 33, 178–191 (2006). https://doi.org/10.1007/s00269-006-0066-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0066-1