Abstract
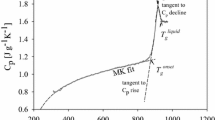
The low-temperature heat capacity (C p ) of KAlSi3O8 with a hollandite structure was measured over the range of 5–303 K with a physical properties measurement system. The standard entropy of KAlSi3O8 hollandite is 166.2±0.2 J mol−1 K−1, including an 18.7 J mol−1 K−1 contribution from the configurational entropy due to disorder of Al and Si in the octahedral sites. The entropy of K2Si4O9 with a wadeite structure (Si-wadeite) was also estimated to facilitate calculation of phase equilibria in the system K2O–Al2O3–SiO2. The calculated phase equilibria obtained using Perple_x are in general agreement with experimental studies. Calculated phase relations in the system K2O–Al2O3–SiO2 confirm a substantial stability field for kyanite–stishovite/coesite–Si-wadeite intervening between KAlSi3O8 hollandite and sanidine. The upper stability of kyanite is bounded by the reaction kyanite (Al2SiO5) = corundum (Al2O3) + stishovite (SiO2), which is located at 13–14 GPa for 1,100–1,400 K. The entropy and enthalpy of formation for K-cymrite (KAlSi3O8·H2O) were modified to better fit global best-fit compilations of thermodynamic data and experimental studies. Thermodynamic calculations were undertaken on the reaction of K-cymrite to KAlSi3O8 hollandite + H2O, which is located at 8.3–10.0 GPa for the temperature range 800–1,600 K, well inside the stability field of stishovite. The reaction of muscovite to KAlSi3O8 hollandite + corundum + H2O is placed at 10.0–10.6 GPa for the temperature range 900–1,500 K, in reasonable agreement with some but not all experiments on this reaction.






Similar content being viewed by others
References
Akaogi M (2000) Clues from a shocked meteorite. Science 287:1602–1603
Akaogi M, Kamii N, Kishi A, Kojitani H (2004) Calorimetric study on high-pressure transitions in KAlSi3O8. Phys Chem Minerals 31:85–91
Carpenter MA, Salje EKH (1994) Thermodynamics of non-convergent cation ordering in minerals: III. Order parameter coupling in potassium feldspar. Am Mineral 79:1084–1098
Chopin C (2003) Ultrahigh-pressure metamorphism: tracing continental crust into the mantle. Earth Planet Sci Lett 212:1–14
Connolly JAD (1990) Multivariable phase diagrams: an algorithm based on generalized thermodynamics. Am J Sci 290:666–718
Connolly JAD, Kerrick DM (1987) An algorithm and computer program for calculating composition phase diagrams. CALPHAD 11:1–55
Dachs E, Bertoldi C (2005) Precision and accuracy of the heat-pulse calorimetric technique: low temperature heat capacities of milligram-sized synthetic mineral samples. Eur J Mineral 17:251–259
Domanik KJ, Holloway JR (1996) The stability and composition of phengitic muscovite and associated phases from 5.5 to 11 GPa: implications for deeply subducted sediments. Geochim Cosmochim Acta 60:4133–4150
Domanik KJ, Holloway JR (2000) Experimental synthesis and phase relations of phengitic muscovite from 6.5 to 11 GPa in a calcareous metapelite from the Dabie Mountains, China. Lithos 52:51–77
Edwards RL, Essene EJ (1998) Pressure, temperature and C–O–H fluid fugacities across the amphibolite-granulite facies transition. NW, Adirondack Mtns., NY. J Petrol 29:39–73
Fasshauer DW, Chatterjee ND, Marler B (1997) Synthesis, structure, thermodynamic properties and stability relations of K-cymrite, KAlSi3O8·H2O. Phys Chem Minerals 24:455–462
Fasshauer DW, Wunder B, Chatterjee ND, Höhne GWH (1998) Heat capacity of wadeite-type K2Si4O9 and the pressure-induced stable decomposition of K-feldspar. Contrib Mineral Petrol 131:210–218
Faust J, Knittle E (1994) The equation of state, amorphization, and high-pressure phase diagram of muscovite. J Geophys Res 99:19785–19792
Geisinger KL, Ross NL, McMillan P, Navrotsky A (1987) Potassium silicate (K2Si4O9): energetics and vibrational spectra of glass, sheet silicate, and wadeite-type phases. Am Mineral 72:984–994
Gillet P, Chen M, Dubrovinsky L, El Goresy A (2000) Natural NaAlSi3O8-hollandite in the shocked Sixiangkou meteorite. Science 287:1633–1636
Harlow GE, Davies R (2004) Status report on stability of K-rich phases at upper-mantle conditions. Lithos 77:647–653
Holland TJB (1989) Dependence of entropy on volume for silicate and oxide minerals: a review and a predictive model. Am Mineral 74:5–13
Holland TJB, Powell R (1991) A compensated-Redlich-Kwong (CORK) equation for volumes and fugacities of CO2 and H2O in the range 1 bar to 50 kbar and 100–1600°C. Contrib Mineral Petrol 109:265–273
Holland TJB, Powell R (1998) An internally consistent thermodynamic data set for phases of petrological interest. J Metam Geol 16:309–343
Hwang SL, Shen P, Chu H-T, Yui T-F, Liou JG, Sobolev NV, Zhang R-Y, Shatsky VS, Zayachkovsky AA (2004) Kokchetavite: a new potassium-feldspar polymorph from the Kokchetav ultrahigh-pressure terrane. Contrib Mineral Petrol 148:380–389
Irifune T, Ringwood RE, Hibberson WO (1994) Subduction of continental crust and terrigenous and pelagic sediments: and experimental study. Earth Planet Sci Lett 126:351–368
Kaneko Y, Maruyama S, Terabayashi M, Yamamoto H, Ishikawa M, Anma R, Parkinson CD, Ota T, Nakajima Y, Katayama I, Yamamoto J, Yamauchi K (2000) Geology of the Kokchetav UHP-HP metamorphic belt, Northern Kazakhstan. Island Arc 9:264–283
Kimura M, Chen M, Yoshida Y, El Goresy A, Ohtani E (2003) Back-transformation of high-pressure phases in a shock melt vein of an H-chondrite during atmospheric passage: implications for the survival of high-pressure phases after decompression. Earth Planet Sci Lett 217:141–150
Kinomura N, Kume N, Koizumi M (1975) Stability of K2Si4O9 with wadeite type structure. In: Proceedings of the 4th international conference on high pressure sci tech, pp 211–214
Kinomura N, Koizumi M, Kume S (1977) Crystal structures of phases produced by disproportionation of K-feldspar under pressure. In: Manghnani MH, Akimoto S (eds) High-pressure research: application in geophysics. Academic, New York, pp 183–189
Konzett J, Fei Y (2000) Transport and storage of potassium in the earth’s upper mantle and transition zone: an experimental study to 23 GPa in simplified and natural bulk compositions. J Petrol 41:583–603
Langenhorst F, Poirier JP (2000) “Eclogitic” minerals in a shocked basaltic meteorite. Earth Planet Sci Lett 176:259–265
Lashley JC, Hundley MF, Migliori A, Sarrao JL, Pagliuso PG, Darling TW, Jaime M, Cooley JC, Hults WL, Morales L, Thoma DJ, Smith JL, Boerio-Goates J, Woodfield BF, Stewart GR, Fisher RA, Phillips NE (2003) Critical examination of heat capacity measurements made on a quantum design physical property measurement system. Cryogenics 43:369–378
Liu L (1978) High-pressure phase transitions of kalsilite and related potassium bearing aluminosilicates. Geochem J 12:275–277
Massonne H-J (1992) Evidence for low-temperature ultrapotassic siliceous fluids in subduction zone environments from experiments in the system K2O–MgO–Al2O3–SiO2–H2O (KMASH). Lithos 28:421–434
Massonne H-J, Nasdala L (2003) Characterization of an early metamorphic stage through inclusions in zircon of a diamondiferous quartzofeldspathic rock from the Erzgebirge, Germany. Am Mineral 88:883–889
Nishiyama N, Rapp RP, Irifune T, Sanehira T, Yamazaki D, Funakoshi K (2005) Stability and P–V–T equation of state of KAlSi3O8-hollandite determined by in situ X-ray observations and implications for dynamics of subducted continental crust material. Phys Chem Mineral 32:627–637
Ono S (1998) Stability limits of hydrous minerals in sediment and mid-ocean ridge basalt compositions: implications for water transport in subduction zones. J Geophys Res 103:18253–18267
Prewitt CT, Downs RT (1998) High-pressure crystal chemistry. Rev Mineral 37:283–312
Ringwood AE (1975) Composition and petrology of the Earth’s mantle. McGraw-Hill, New York, 618 pp
Ringwood AE, Reid AF, Wadsley AD (1967) High-pressure KAlSi3O8, an alumino-silicate with sixfold coordination. Acta Crystallogr 23:1093–1095
Robie RA, Hemingway BS (1995) Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures. US Geological Survey 2131, 461 pp
Schertl H-P, Schreyer W, Chopin C (1991) The pyrope-coesite rocks and their country rocks at Parigi, Dora Maira massif, western Alps: detailed petrography, mineral chemistry and PT-path. Contrib Mineral Petrol 108:1–21
Schmidt M (1996) Experimental constraints on recycling of potassium from subducted oceanic crust. Science 272:1927–1930
Schmidt MW, Poly S, Comodi P, Zanazzi PF (1997) High-pressure behavior of kyanite: decomposition of kyanite into stishovite and corundum. Am Mineral 82:460–466
Seki Y, Kennedy GC (1964) The breakdown of potassium feldspar, KAlSi3O8, at high temperatures and high pressures. Am Mineral 49:1688–1706
Sekine T, Rubin AM, Ahrens TJ (1991) Shock wave equation of state of muscovite. J Geophys Res 96:19675–19680
Sharp ZD, Essene EJ, Hunziker JC (1993) Stable isotope geochemistry and phase equilibria of coesite-bearing whiteschists, Dora Maira massif, western Alps. Contrib Mineral Petrol 114:1–12
Sueda Y, Irifune T, Nishiyama N, Rapp RP, Ferroir T, Onozawa T, Yagi T, Merkel S, Miyajima N, Funakoshi K (2004) A new high-pressure form of KAlSi3O8 under lower mantle conditions. Geophys Res Lett 31:L23612. DOI 10.1029/2004GL021156
Swanson DK, Prewitt CT (1983) The crystal structure of K2SiVI–Si IV3 O9. Am Mineral 68:581–585
Swanson DK, Prewitt CT (1986) Anharmonic thermal motion in K2SiVI–Si IV3 O9. Eos 67:369
Thompson P (1994) The sanidine–‘sanidine hydrate’ reaction boundary. Mineral Mag 58A:897
Thompson P, Parsons I, Graham CM, Jackson B (1998) The breakdown of potassium feldspar at high water pressures. Contrib Mineral Petrol 130:176–186
Tomioka N, Mori H, Fujino K (2000) Shock-induced transition of NaAlSi3O8 feldspar into a hollandite structure in a L6 chondrite. Geophys Res Lett 278:3997–4000
Tutti F, Dubrovinsky LS, Saxena SK, Carlson S (2001) Stability of KAlSi3O8 hollandite-type structure in the Earth’s lower mantle conditions. Geophys Res Lett 28:2735–2738
Urakawa S, Kondo T, Igawa N, Shimomura O, Ohno H (1994) Synchrotron radiation study on the high-pressure and high-temperature phase relations of KAlSi3O8. Phys Chem Mineral 21:387–391
Valley JW, Bohlen SR, Essene EJ, Lamb W (1990) Metamorphism in the Adirondacks. II. The role of fluids. J Petrol 31:555–596
Wang W, Takahashi E (1999) Subsolidus and melting experiments of a K-rich basaltic composition to 27 GPa: implication for the behavior of potassium in the mantle. Am Mineral 84:357–361
Yagi A, Akimoto S (1976) Direct determination of coesite-stishovite transition by in situ X-ray measurements. Tectonophys 35:259–586
Yagi A, Suzuki T, Akaogi M (1994) High pressure transitions in the system KAlSi3O8–NaAlSi3O8. Phys Chem Minerals 21:12–17
Yamada H, Matsui Y, Ito E (1984) Crystal-chemical characterization of KAlSi3O8 with hollandite structure. Mineral J 12:29–34
Yoshida D, Hirajima T, Ishiwatari A (2004) Pressure-temperature path recorded in the Yangkou garnet peridotite, in Su–Lu ultrahigh-pressure metamorphic belt, eastern China. J Petrol 45:1125–1145
Zhang J, Ko J, Hazen RM, Prewitt CT (1993) High-pressure crystal chemistry of KAlSi3O8 hollandite. Am Mineral 78:493–499
Zhang J, Li B, Utsumi W, Liebermann RC (1996) In situ X-ray observations of the coesite–stishovite transition: reversed phase boundary and kinetics. Phys Chem Minerals 23:1–10
Acknowledgements
The authors are grateful to C. Manning of UCLA for providing 2 g of sanidine glass for use in this study. They also thank Z. Page and C. Henderson for their help in EMPA analysis, and R.C. Rouse for his help with XRD measurements. The authors acknowledge M. Akaogi and J. Konzett for their constructive reviews of the manuscript. This work was partly supported by Scott Turner Research Grant by the Department of Geological Sciences, University of Michigan to the senior author, and by NSF grants EAR96-28196, 99-11352, 00-87448 and 05-37068 to EJE. NSF grants EAR 03-10142 and 00-79827 to M. Hirschmann for the multianvil device at the University of Minnesota, and support of the Austrian granting agency for the PPMS at the University of Salzburg (grant P15880-N11) are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yong, W., Dachs, E., Withers, A.C. et al. Heat capacity and phase equilibria of hollandite polymorph of KAlSi3O8 . Phys Chem Minerals 33, 167–177 (2006). https://doi.org/10.1007/s00269-006-0063-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0063-4