Abstract
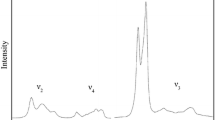
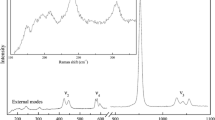
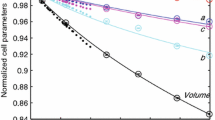
Raman spectra of monoclinic Fo90 hydrous wadsleyite with 2.4 wt% H2O have been measured in a diamond-anvil cell with helium as a pressure-transmitting medium to 58.4 GPa at room temperature. The most intense, characteristic wadsleyite modes, the Si–O–Si symmetric stretch at 721 cm−1 and the symmetric stretch of the SiO3 unit at 918 cm−1, shift continuously to 58.4 GPa showing no evidence of a first order change in the crystal structure despite compression well beyond the stability field of wadsleyite in terms of pressure. The pressure dependence of these two modes is nearly identical for Fo90 hydrous and Fo100 anhydrous wadsleyite. A striking feature in the high-pressure Raman spectra of Fo90 hydrous wadsleyite is the appearance of new Raman modes above 9 GPa in the mid-frequency range (300–650 cm−1 at 1-bar and shifted to 500–850 cm−1 at 58.4 GPa) accompanied by a significant growth in their intensities under further compression. In the OH stretching frequency range Fo90 hydrous wadsleyite exhibits a larger number of modes than the Mg end-member phase. The higher number of modes may be due to either additional protonation sites or simply that we observe a different subset of all possible OH modes for each sample. The high-pressure behaviour of the OH stretching modes of Fo90 and Fo100 hydrous wadsleyite is consistent: OH stretching modes with frequencies <3,530 cm−1 decrease with increasing pressure whereas the higher-frequency OH modes show a close to constant pressure dependence to at least 13.2 GPa. The approximately constant pressure dependence of the OH modes above 3,530 cm−1 is consistent with protons being located at the O1···O edges around M3.





Similar content being viewed by others
References
Bolfan-Casanova N, Keppler H, Rubie DC (2000) Water partitioning between nominally anhydrous minerals in the MgO–SiO2–H2O system up to 24 GPa: implications for the distribution of water in the Earth’s mantle. Earth Planet Sci Lett 182:209–221
McCammon CA, Frost DJ, Smyth JR, Laustsen HMS, Kawamoto T, Ross NL, van Aken PA (2004) Oxidation state of iron in hydrous mantle phases: implications for subduction and mantle oxygen fugacity. Phys Earth Planet Int 143–144:157–169
Chopelas A (1991) Thermal properties of β-Mg2SiO4 at mantle pressures derived from vibrational spectroscopy: implications for the nature of the 400-km seismic discontinuity. J Geophys Res 96 (B7):11817–11829
Cynn H, Hofmeister AM (1994) High-pressure IR spectra of lattice modes and OH vibrations in Fe-bearing wadsleyite. J Geophys Res 99(B9):17717–17727
Downs JW (1989) Possible sites for protonation in β-Mg2SiO4 from an experimentally derived electrostatic potential. Am Mineral 74:1124–1129
Finger LW, Hazen RM, Zhang J, Ko J, Navrotsky A (1993) The effect of Fe on the crystal structure of wadsleyite β-(Mg1-x Fe x )2SiO4, 0.00≤x≤0.40. Phys Chem Minerals 19: 361–368
Hazen RM, Navrotsky A (1996) Effects of pressure on order–disorder reactions. Am Mineral 81:1021–1035
Hazen RM, Weinberger MB, Yang H, Prewitt CT (2000a) Comparative high-pressure crystal chemistry of wadsleyite, β-(Mg1-x Fe x )SiO4, with x=0 and 0.25. Am Mineral 85: 770–777
Hazen RM, Yang H, Prewitt CT (2000b) High-pressure crystal chemistry of Fe3+-wadsleyite β-Fe2.33Si0.67O4. Am Mineral 85:778–783
Hazen RM, Zhang J, Ko J (1990) Effects of Fe/Mg on the compressibility of synthetic wadsleyite: β-(Mg1-x Fe x )SiO4 (x≤0.25). Phys Chem Minerals 17:416–419
Horiuchi H, Sawamoto H (1981) β-Mg2SiO4: single-crystal X-ray diffraction study. Am Mineral 66:568–575
Inoue T, Yurimoto H, Kudoh Y (1995) Hydrous modified spinel, Mg1,75SiH0,5O4: a new water reservoir in the mantle transition region. Geophys Res Lett 22(2):117–120
Jacobsen SD, Demouchy S, Frost DJ, Boffa Ballaran T, Kung J (2005) A systematic study of OH in hydrous wadsleyite from polarized FTIR spectroscopy and single-crystal X-ray diffraction: Oxygen sites for hydrogen storage in Earth’s interior. Am Mineral 90: 61–70. DOI: 10.2138/am.2005.1624
Jephcoat AP, Mao HK, Bell PM (1987) Operation of the megabar diamond-anvil cell. In: Ulmer GC, Barnes HL (eds) Hydrothermal experimental techniques, chap 19. Wiley-Interscience, New York, pp 469–506
Keppler H, Smyth JR (2005) Optical and near infrared spectra of ringwoodite to 21.5 GPa: implications for radiative heat transport in the mantle. Am Mineral 90: 1209–1212
Kleppe AK, Jephcoat AP, Olijnyk H, Slesinger AE, Kohn SC, Wood BJ (2001) Raman spectroscopic study of hydrous wadsleyite (β-Mg2SiO4) to 50 GPa. Phys Chem Minerals 28:232–241
Kleppe AK, Jephcoat AP, Smyth JR (2002a) Raman spectroscopic study of hydrous γ-Mg2SiO4 to 56.6 GPa. Phys Chem Minerals 29(7):473–476
Kleppe AK, Jephcoat AP, Smyth JR, Frost DJ (2002b) On protons, iron and the high-pressure behavior of ringwoodite. Geophys Res Lett 29(21):17,1–17,4. DOI: 10.1029/2002GL015276
Kohlstedt DL, Keppler H, Rubie DC (1996) Solubility of water in the α, β and γ phases of (Mg,Fe)2SiO4. Contrib Mineral Petrol 123:345–357
Kohn SC, Brooker RA, Frost DJ, Slesinger AE, Wood BJ (2002) Ordering of hydroxyl defects in hydrous wadsleyite (β-Mg2SiO4). Am Mineral 87:293–301
Kudoh Y, Inoue T (1998) Effect of pressure on the crystal structure of hydrous wadsleyite, Mg1,75SiH0,5O4 In: Properties of earth and planetary materials at high pressure and temperature. Geophys Monogr 101:517–520, AGU
Kudoh Y, Inoue T (1999) Mg-vacant structural modules and dilution of the symmetry of hydrous wadsleyite β-Mg2-x SiH2x O4 with 0.00≤x≤0.25. Phys Chem Minerals 26:382–388
Kudoh Y, Inoue T, Arashi H (1996) Structure and crystal chemistry of hydrous wadsleyite, Mg1.75SiH0.5O4: possible hydrous magnesium silicate in the mantle transition zone. Phys Chem Minerals 23:461–469
Liu L-G, Mernagh TP, Lin CC, Xu J, Inoue T (1998) Raman spectra of hydrous β-Mg2SiO4 at various pressures and temperatures In: Properties of earth and planetary materials at high pressure and temperature. Geophys Monogr 101:523–530, AGU
Mao H-K, Bell PM, Shaner JW, Steinberg DJ (1978) Specific volume measurements of Cu, Mo, Pd, and Ag and calibration of the ruby R1 fluorescence pressure gauge from 0.06 to 1 Mbar. J Appl Phys 49(6):3276–3283
McMillan PF, Akaogi M, Sato RK, Poe B, Foley J (1991) Hydroxyl groups in β-Mg2SiO4. Am Mineral 76:354–360
Mernagh TP, Liu L-G (1996) Raman and infrared spectra of hydrous β-Mg2SiO4. Can Mineral 34:1233–1240
Parise JB, Leinenweber K, Weidner DJ, Tan K, von Dreele RB (1994) Pressure-induced H bonding: neutron diffraction study of brucite, Mg(OD)2, to 9.3 GPa. Am Mineral 79:193–196
Smyth JR (1987) β-Mg2SiO4: a potential host for water in the mantle? Am Mineral 72:1051–1055
Smyth JR (1994) A crystallographic model for hydrous wadsleyite (β-Mg2SiO4): an ocean in the Earth’s interior? Am Mineral 79:1021–1024
Smyth JR, Kawamoto T (1997) Wadsleyite II: a new high pressure hydrous phase in the peridotite-H2O system. Earth Planet Sci Lett 146:E9–E16
Smyth JR, Kawamoto T, Jacobsen SD, Swope RJ, Hervig RL, Holloway JR (1997) Crystal structure of monoclinic hydrous wadsleyite [β-(Mg,Fe)2SiO4]. Am Mineral 82:270–275
Smyth JR, Holl CM, Langenhorst F, Laustsen HM, Rossman GR, Kleppe AK, McCammon CA, Kawamoto T, van Aken PA (2005) Crystal chemistry of wadsleyite II and water in the Earth’s interior. Phys Chem Minerals 31:691–705
Smyth JR, Nguyen CK, Frost DJ, McCammon CA, Langenhorst F, Bolfan-Casanova N (2000) Hydration of Fe-bearing silicate spinels and spinelloids. EOS (Trans. Am. Geophys. Union) 81:F1274
Wright K, Catlow CRA (1996) Calculations on the energetics of water dissolution in wadsleyite. Phys Chem Minerals 23:38–41
Yusa H, Inoue T (1997) Compressibility of hydrous wadsleyite (β-phase) in Mg2SiO4 by high-pressure X-ray diffraction. Geophys Res Lett 24(14):1831–1834
Acknowledgement
This work was supported by Natural Environment Research Council fellowship NER/I/S/2001/00723 and grant NER/B/S/2003/00258 to A.K.K., and Natural Environment Research Council grants GT59801ES, and GR3/10912 to A.P.J.. This research was also supported by the U.S. National Science Foundation grant EAR 03–36611 to J.R.S., the Bayerisches Geoinstitut Visitor Program, and the Alexander von Humboldt Foundation. We thank two anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kleppe, A.K., Jephcoat, A.P. & Smyth, J.R. High-pressure Raman spectroscopic study of Fo90 hydrous wadsleyite. Phys Chem Minerals 32, 700–709 (2006). https://doi.org/10.1007/s00269-005-0048-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-005-0048-8