Abstract
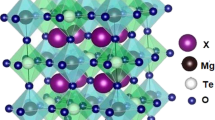
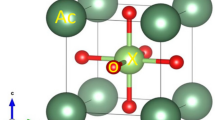
Phase transitions in MgGeO3 and ZnGeO3 were examined up to 26 GPa and 2,073 K to determine ilmenite–perovskite transition boundaries. In both systems, the perovskite phases were converted to lithium niobate structure on release of pressure. The ilmenite–perovskite boundaries have negative slopes and are expressed as P(GPa)=38.4–0.0082T(K) and P(GPa)=27.4−0.0032T(K), respectively, for MgGeO3 and ZnGeO3. Enthalpies of SrGeO3 polymorphs were measured by high-temperature calorimetry. The enthalpies of SrGeO3 pseudowollasonite–walstromite and walstromite–perovskite transitions at 298 K were determined to be 6.0±8.6 and 48.9±5.8 kJ/mol, respectively. The calculated transition boundaries of SrGeO3, using the measured enthalpy data, were consistent with the boundaries determined by previous high-pressure experiments. Enthalpy of formation (ΔH f°) of SrGeO3 perovskite from the constituent oxides at 298 K was determined to be −73.6±5.6 kJ/mol by calorimetric measurements. Thermodynamic analysis of the ilmenite–perovskite transition boundaries in MgGeO3 and ZnGeO3 and the boundary of formation of SrSiO3 perovskite provided transition enthalpies that were used to estimate enthalpies of formation of the perovskites. The ΔH f° of MgGeO3, ZnGeO3 and SrSiO3 perovskites from constituent oxides were 10.2±4.5, 33.8±7.2 and −3.0±2.2 kJ/mol, respectively. The present data on enthalpies of formation of the above high-pressure perovskites were combined with published data for A2+B4+O3 perovskites stable at both atmospheric and high pressures to explore the relationship between ΔH f° and ionic radii of eightfold coordinated A2+ (R A) and sixfold coordinated B4+ (R B) cations. The results show that enthalpy of formation of A2+B4+O3 perovskite increases with decreasing R A and R B. The relationship between the enthalpy of formation and tolerance factor ( \( t = {\left( {R_{{\text{A}}} + R_{{\text{o}}} } \right)}/{\sqrt {\text{2}} }{\left( {R_{{\text{B}}} + R_{{\text{o}}} } \right)}, \) R o: O2− radius) is not straightforward; however, a linear relationship was found between the enthalpy of formation and the sum of squares of deviations of A2+ and B4+ radii from ideal sizes in the perovskite structure. A diagram showing enthalpy of formation of perovskite as a function of A2+ and B4+ radii indicates a systematic change with equienthalpy curves. These relationships of ΔH f° with R A and R B can be used to estimate enthalpies of formation of perovskites, which have not yet been synthesized.






Similar content being viewed by others
References
Akaogi M, Ito E (1993) Refinement of enthalpy measurement of MgSiO3 perovskite and negative pressure–temperature slopes for perovskite-forming reactions. Geophys Res Lett 20:1839–1842
Akaogi M, Navrotsky A (1985) Calorimetric study of high-pressure polymorphs of MnSiO3. Phys Chem Miner 12:317–323
Akaogi M, Navrotsky A (1987) Calorimetric study of high-pressure phase transitions among the CdGeO3 polymorphs (pyroxenoid, garnet, ilmenite, and perovskite structures). Phys Chem Miner 14:435–440
Akaogi M, Suzuki T, Kojima R, Honda T, Ito E, Nakai I (1998) Enthalpy and density measurements of pressure-amorphized GeO2 quartz. Geophys Res Lett 25:3635–3638
Akaogi M, Yano M, Tejima Y, Iijima M, Kojitani H (2004) High-pressure transitions of diopside and wollastonite: phase equilibria and thermochemistry of CaMgSi2O6, CaSiO3 and CaSi2O5–CaTiSiO5 system. Phys Earth Planet Int 143–144:145–156
Akaogi M, Yusa H, Shiraishi K, Suzuki T (1995) Thermodynamic properties of α-quartz, coesite, and stishovite and equilibrium phase relations at high pressures and high temperatures. J Geophys Res 100:22337–22347
Andrault D, Itie JP, Farges F (1996) High-temperature structural study of germanate perovskites and pyroxenoids. Am Mineral 81:822–832
Ashida T, Miyamoto Y, Kume S (1985) Heat capacity, compressibility and thermal expansion coefficient of ilmenite-type MgSiO3. Phys Chem Miner 12:129–131
Bohlen SR, Boettcher AL (1982) The quartz-coesite transformation: a precise determination and the effects of other components. J Geophys Res 87:7073–7078
Fujino K, Izumi H, Shizuka Y, Takafuji N, Nagai T, Hamane D (2004) Stability and structural change of various silicate perovskites. Abst Ann Meet Jpn Soc High Press Sci Tech 14:132
Funamori N, Yagi T, Utsumi W, Kondo T, Uchida T, Funamori M (1996) Thermoelastic properties of MgSiO3 perovskite determined by in situ X ray observation up to 30 GPa and 2000 K. J Geophys Res 101:8257–8269
Goldschmidt VM (1926) Die Gesetze der Krystallochemie. Naturwissenschaften 14:477–485
Gramsch SA, Morss LR (1995) Standard molar enthalpies of formation of PrO2 and SrPrO3: the unusual thermodynamic stability of APrO3 (A=Sr, Ba). J Chem Thermodyn 27:551–560
Hattori T, Matsuda T, Tsuchiya T, Nagai T, Yamanaka T (1999) Clinopyroxene-perovskite phase transition of FeGeO3 under high pressure and room temperature. Phys Chem Miner 26:212–216
Ito E, Matsui Y (1979) High-pressure transformations in silicates, germanates, and titanates with ABO3 stoichiometry. Phys Chem Miner 4:265–273
Ito E, Takahashi E (1989) Postspinel transformations in the system Mg2SiO4–Fe2SiO4 and some geophysical implications. J Geophys Res 94:10637–10646
Ko J, Brown NE, Navrotsky A, Prewitt CT, Gasparik T (1989) Phase equilibrium and calorimetric study of the transition of MnTiO3 from the ilmenite to the lithium niobate structure and implications for the stability field of perovskite. Phys Chem Miner 16:727–733
Kojitani H, Kido M, Akaogi M (2005) Rietveld analysis of a new high-pressure strontium silicate SrSi2O5. Phys Chem Miner 32:290–294
Kojitani H, Navrotsky A, Akaogi M (2001) Calorimetric study of perovskite solid solutions in the CaSiO3–CaGeO3 system. Phys Chem Miner 28:413–420
Leinenweber K, Navrotsky A, McMillan P, Ito E (1989) Transition enthalpies and entropies of high pressure zinc metasilicates and zinc metagermanates. Phys Chem Miner 16:799–808
Leinenweber K, Wang Y, Yagi T, Yusa H (1994) An unquenchable perovskite phase of MgGeO3 and comparison with MgSiO3 perovskite. Am Mineral 79:197–199
Leinenweber K, Utsumi W, Tsuchida Y, Yagi T, Kurita K (1991) Unquenchable high-pressure perovskite polymorphs of MnSnO3 and FeTiO3. Phys Chem Miner 18:244–250
Linton JA, Fei Y, Navrotsky A (1999) The MgTiO3–FeTiO3 join at high pressure and temperature. Am Mineral 84:1595–1603
Liu L (1977) The post-spinel phases of twelve silicates and gernamates. In: Manghnani MH, Akimoto S (eds) High-pressure research: applications in geophysics. Academic Press, New York, NY, pp 245–253
Morishima H, Kato T, Suto M, Ohtani E, Urakawa S, Utsumi W, Shimomura O, Kikegawa T (1994) The phase boundary between α- and β-Mg2SiO4 determined by in situ X-ray observation. Science 265:1202–1203
Navrotsky A (1971) Thermodynamics of formation of the silicates and germanates of some divalent transition metals and of magnesium. J Inorg Nucl Chem 33:4035–4050
Navrotsky A (1980) Lower mantle phase transitions may generally have negative pressure-temperature slopes. Geophys Res Lett 7:709–711
Navrotsky A (1981) Energetics of phase transitions in AX, ABO3, and AB2O4 compounds. In: O’Keeffe M, Navrotsky A (eds) Structure and bonding in crystals, vol 2. Academic Press, pp 71–93
Navrotsky A (1998) Energetics and crystal chemical systematics among ilmenite, lithium niobate, and perovskite structures. Chem Mater 10:2787–2793
Ono S, Katsura T, Ito E, Kanzaki M, Yoneda A, Walter MJ, Urakawa S, Utsumi W, Funakoshi K (2001) In situ observation of ilmenite–perovskite phase transition in MgSiO3 using synchrotron radiation. Geophys Res Lett 28:835–838
Richet P (1990) GeO2 vs. SiO2: Glass transitions and thermodynamic properties of polymorphs. Phys Chem Miner 17:79–88
Ringwood AE, Seabrook M (1962) High-pressure phase transition in MgGeO3 from pyroxene to corundum structure. J Geophys Res 67:1690–1691
Ringwood AE, Seabrook M (1963) High-pressure phase transformations in germanate pyroxenes and related compounds. J Geophys Res 68:4601–4609
Robie R, Hemingway BS (1995) Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 pascals) pressure and at higher temperatures. US Geol Surv 2131:461
Ross NL, Akaogi M, Navrotsky A, Susaki J, McMillan P (1986) Phase transitions among CaGeO3 polymorphs (wollastonite, garnet, and perovskite structures): studies by high pressure synthesis, high temperature calorimetry, and vibrational spectroscopy and calculation. J Geophys Res 91:4685–4696
Ross NL, Ko J, Prewitt CT (1989) A new phase transition in MnTiO3: LiNbO3–perovskite structure. Phys Chem Miner 16:621–629
Ross NL, Leinenweber K (1990) Single crystal structure refinement of high-pressure ZnGeO3 ilmenite. Z Krist 191:93–104
Ross NL, Navrotsky A (1988) Study of the MgGeO3 polymorphs (orthopyroxene, clinopyroxene, and ilmenite structures) by calorimetry, spectroscopy, and phase equilibria. Am Mineral 73:1355–1365
Sato Y, Akimoto S (1979) Hydrostatic compression of perovskite phase of SrGeO3. High Press Sci Tech 2:91–96
Sato Y, Ito E, Akimoto S (1977) Hydrostatic compression of ilmenite phase of ZnSiO3 and MgGeO3. Phys Chem Miner 2:171–176
Shannon RD, Prewitt CT (1969) Effective ionic radii in oxides and fluorides. Acta Cryst B25:925–946
Shimizu Y, Syono Y, Akimoto S (1970) High-pressure transformations in SrGeO3, SrSiO3, BaGeO3 and BaSiO3. High Temp High Press 2:113–120
Suzuki A, Ohtani E, Morishima H, Kubo T, Kanbe Y, Okada T, Terasaki H, Kato T, Kikegawa T (2000) In situ determination of the phase boundary between wadsleyite and ringwoodikte in Mg2SiO4. Geophys Res Lett 27:803–806
Swamy V, Dubrovinsky LS (1997) Thermodynamic data for the phases in the CaSiO3 system. Geochim Cosmochim Acta 61:1181–1191
Syono Y, Akimoto S, Matsui Y (1971) High pressure transformations in zinc silicates. J Solid State Chem 3:369–380
Takayama-Muromachi E, Navrotsky A (1988) Energetics of compounds (A2+B4+O3) with the perovskite structure. J Solid State Chem 72:244–256
Ushakov SV, Cheng J, Navrotsky A, Wu JR, Haile SM (2002) Formation enthalpies of tetravalent lanthanide perovskites by high temperature oxide melt solution calorimetry. Mat Res Symp Proc 718:71–76
Yagi T, Akaogi M, Shimomura O, Suzuki T, Akimoto S (1987) In situ observation of the olivine-spinel phase transformation in Fe2SiO4 using synchrotron radiation. J Geophys Res 92:6207–6213
Yusa H, Akaogi M, Sata N, Kojitani H, Kato Y, Ohishi Y (2005) Unquenchable hexagonal perovskite in high pressure polymorphs of strontium silicates. Am Mineral 90:1017–1020
Zhang J, Li B, Utsumi W, Liebermann RC (1996) In situ X-ray observations of the coesite–stishovite transition: reversed phase boundary and kinetics. Phys Chem Miner 23:1–10
Acknowledgements
We are grateful to Y. Inaguma and K. Fujino for their valuable discussions. We thank Y. Mizobuchi for some of the calorimetric measurements on zinc compounds. The constructive comments by two anonymous reviewers were very helpful in improving the manuscript. This work was supported in part by the Grant-in-Aid from the Japan Society for the Promotion of Science and by High-Technology Research Center Project from the Japanese Ministry of Education, Science, Sports and Culture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akaogi, M., Kojitani, H., Yusa, H. et al. High-pressure transitions and thermochemistry of MGeO3 (M=Mg, Zn and Sr) and Sr-silicates: systematics in enthalpies of formation of A2+B4+O3 perovskites. Phys Chem Minerals 32, 603–613 (2005). https://doi.org/10.1007/s00269-005-0034-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-005-0034-1