Abstract
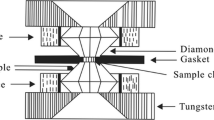
An equation of state for Mg(OH)2 brucite under high-pressure and high-temperature conditions has been obtained by measuring temperature dependence of volume up to 600 K at ambient pressure and pressure dependence of volume up to 16 GPa at 300, 473, 673, and 873 K with in situ X-ray diffraction. Pressure dependence of entropy of brucite has been calculated with thermal expansion coefficient and volume which are derived from the present EoS. This dependence indicates that generation of secondary OH dipoles affects entropy. The OH dipoles probably appear around 2 GPa and the number seems not to change over 8 GPa at 300 K.
Similar content being viewed by others
Acknowledgments.
The authors thank to Dr. A. Pavese and an anonymous referee for their valuable comments and careful reading. The authors appreciate the help of Mr. H. Takebe and Mr. H. Arima in situ X-ray measurements under pressure. The X-ray diffraction studies at BL04B1 in SPring-8 were performed with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (proposal no. 2001B0057-ND-np). This research is partially supported by JSPS research fellowships for young scientists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukui, H., Ohtaka, O., Suzuki, T. et al. Thermal expansion of Mg(OH)2 brucite under high pressure and pressure dependence of entropy. Phys Chem Minerals 30, 511–516 (2003). https://doi.org/10.1007/s00269-003-0353-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00269-003-0353-z