Abstract
Purpose
Peritoneal carcinomatosis from appendiceal goblet cell carcinoma (A-GCC) is a rare and aggressive form of appendiceal tumor. Cytoreductive surgery (CRS) and hyperthermic intra peritoneal chemotherapy (HIPEC) was reported as an interesting alternative regarding survival compared to surgery without HIPEC and/or systemic chemotherapy. Our aim was to evaluate the impact of CRS and HIPEC for patients presenting A-GCC through an international registry.
Methods
A prospective multicenter international database was retrospectively searched to identify all patients with A-GCC tumor and peritoneal metastases who underwent CRS and HIPEC through the Peritoneal Surface Oncology Group International (PSOGI). The post-operative complications, long-term results, and principal prognostic factors were analyzed.
Results
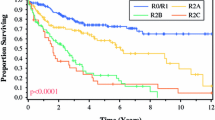
The analysis included 83 patients. After a median follow-up of 47 months, the median overall survival (OS) was 34.6 months. The 3- and 5-year OS was 48.5% and 35.7%, respectively. Patients who underwent complete macroscopic CRS had a significantly better survival than those treated with incomplete CRS. The 5-year OS was 44% and 0% for patients who underwent complete, and incomplete CRS, respectively (HR 9.65, p < 0.001). Lymph node involvement and preoperative chemotherapy were also predictive of a worse prognosis. There were 3 postoperative deaths, and 30% of the patients had major complications.
Conclusion
CRS and HIPEC may increase long-term survival in selected patients with peritoneal metastases of A-GCC origin, especially when complete CRS is achieved. Ideally, randomized control trials or more retrospective data are needed to confirm CRS and HIPEC as the gold standard in this pathology.


Similar content being viewed by others
References
Olsen IH, Holt N, Langer SW et al (2015) Goblet cell carcinoids: characteristics of a Danish cohort of 83 patients. PLoS ONE 10:e0117627
Pahlavan PS, Kanthan R (2005) Goblet cell carcinoid of the appendix. World J Surg Oncol 3:36
Plockinger U, Couvelard A, Falconi M et al (2008) Consensus guidelines for the management of patients with digestive neuroendocrine tumours: well-differentiated tumour/carcinoma of the appendix and goblet cell carcinoma. Neuroendocrinology 87:20–30
Toumpanakis C, Standish RA, Baishnab E et al (2007) Goblet cell carcinoid tumors (adenocarcinoid) of the appendix. Dis Colon Rectum 50:315–322
Pham TH, Wolff B, Abraham SC et al (2006) Surgical and chemotherapy treatment outcomes of goblet cell carcinoid: a tertiary cancer center experience. Ann Surg Oncol 13:370–376
McCusker ME, Cote TR, Clegg LX et al (2002) Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer 94:3307–3312
Pape UF, Perren A, Niederle B et al (2012) ENETS consensus guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology 95:135–156
Shaib W, Krishna K, Kim S et al (2016) Appendiceal neuroendocrine, goblet and signet-ring cell tumors: a spectrum of diseases with different patterns of presentation and outcome. Cancer Res Treat 48:596–604
Kanthan R, Saxena A, Kanthan SC (2001) Goblet cell carcinoids of the appendix: immunophenotype and ultrastructural study. Arch Pathol Lab Med 125:386–390
Mahteme H, Sugarbaker PH (2004) Treatment of peritoneal carcinomatosis from adenocarcinoid of appendiceal origin. Br J Surg 91:1168–1173
McConnell YJ, Mack LA, Gui X et al (2014) Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: an emerging treatment option for advanced goblet cell tumors of the appendix. Ann Surg Oncol 21:1975–1982
Cashin P, Nygren P, Hellman P et al (2011) Appendiceal adenocarcinoids with peritoneal carcinomatosis treated with cytoreductive surgery and intraperitoneal chemotherapy: a retrospective study of in vitro drug sensitivity and survival. Clin Colorectal Cancer 10:108–112
Goere D, Malka D, Tzanis D et al (2013) Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg 257:1065–1071
Chia CS, You B, Decullier E et al (2016) Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol 23:1971–1979
Bonnot PE, Piessen G, Kepenekian V et al (2019) Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J Clin Oncol 37:2028–2040
Yan TD, Deraco M, Baratti D et al (2009) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 27:6237–6242
Chua TC, Moran BJ, Sugarbaker PH et al (2012) Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 30:2449–2456
Van Driel W, Sikorska K, Schagen van Leeuwen J et al (2017) A phase 3 trial of hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer. Am Soc Clin Oncol 35:5519–5519
Jacquet P, Sugarbaker PH (1996) Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359–374
Glehen O, Mohamed F, Gilly FN (2004) Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol 5:219–228
Institute NC Common Terminology Criteria for Adverse Events (NCI CTCAE) v4.03. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
Liu Y, Yonemura Y, Levine EA et al (2018) Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for peritoneal metastases from a small bowel adenocarcinoma: multi-institutional experience. Ann Surg Oncol 25:1184–1192
Garin L, Corbinais S, Boucher E et al (2002) Adenocarcinoid of the appendix vermiformis: complete and persistent remission after chemotherapy (folfox) of a metastatic case. Dig Dis Sci 47:2760–2762
Bristow RE, Chi DS (2006) Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol 103:1070–1076
Passot G, Vaudoyer D, Villeneuve L et al (2016) What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: a 25-year experience with 1,125 procedures. J Surg Oncol 113:796–803
Acknowledgements
The authors thank Lorraine Bernard (Pôle Santé Publique, Hospices Civils de Lyon) for help with data analysis. The collaborators of the BIG-RENAPE Working Group include the following: J. Abba (Department of Surgical Oncology, CHU Grenoble University, Grenoble, France); K. Abboud (Department of Surgical Oncology, CHU St Etienne, St Etienne, France); Alyami, M. (Department of Surgical Oncology, Centre Hospitalier Lyon Sud - EMR 3738, Lyon 1 University, Lyon, France); C. Arvieux (Department of Surgical Oncology, CHU Grenoble University, Grenoble, France); N. Bakrin (Department of Surgical Oncology, Centre Hospitalier Lyon Sud - EMR 3738, Lyon 1 University, Lyon, France); J-M. Bereder (Department of Surgical Oncology, CHU L'Archet 2, Nice, France); D. Bouzard (Department of Surgical Oncology, CHU Louis Mourier, Colombes, France); C. Brigand (Department of Surgical Oncology, CHRU Hautepierre, Strasbourg, France); S. Carrère (Department of Surgical Oncology, Institut du Cancer de Montpellier, Montpellier, France); D. Delroeux (Department of Surgical Oncology, CHU Jean Minjoz, Besançon, France); F. Dumont (Department of Surgical Oncology, ICO - René Gauducheau, St Herblain, France); C. Eveno (Department of Surgical Oncology, CHRU Claude Huriez, Lille, France); O. Facy (Department of Surgical Oncology, CHU Dijon, Dijon, France); F. Guyon (Department of Surgical Oncology, Institut Bergonié, Bordeaux, France); G. Ferron (Department of Surgical Oncology, IUCT Oncopole, Toulouse, France); R. Kianmanesh (Department of Surgical Oncology, CHU Robert Debré, Reims, France); R. Lo Dico (Department of Surgical Oncology, CHU Lariboisière, Paris, France); G. Lorimier (Department of Surgical Oncology, CHU Angers, Angers, France); F. Marchal (Department of Surgical Oncology, Institut de Cancérologie de Lorraine, Vandoeuvre-lès-Nancy, France); P. Mariani (Department of Surgical Oncology, Institut Curie, Paris, France); P. Meeus (Department of Surgical Oncology, Centre Léon Bérard, Lyon, France); S. Msika (Department of Surgical Oncology, CHU Louis Mourier, Colombes, France); P. Ortega-Deballon (Department of Surgical Oncology, CHU Dijon, Dijon, France); B. Paquette (Department of Surgical Oncology, CHU Jean Minjoz, Besançon, France); P. Peyrat (Department of Surgical Oncology, Centre Léon Bérard, Lyon, France); N. Pirro (Department of Surgical Oncology, CHU La Timône, Marseille, France); M. Pocard (Department of Surgical Oncology, CHU Lariboisière, Paris, France); J. Porcheron (Department of Surgical Oncology, CHU St Etienne, St Etienne, France); F. Quenet (Department of Surgical Oncology, Institut du Cancer de Montpellier, Montpellier, France); P. Rat (Department of Surgical Oncology, CHU Dijon, Dijon, France); O. Sgarbura (Department of Surgical Oncology, Institut du Cancer de Montpellier, Montpellier, France); E. Thibaudeau (Department of Surgical Oncology, ICO - René Gauducheau, St Herblain, France); J-J. Tuech (Department of Surgical Oncology, CHU Charles Nicolle, Rouen, France); F. Zinzindohoue (Department of Surgical Oncology, Hôpital Européen Georges Pompidou, Paris, France). The collaborators of the PSOGI Working Group included the following: S. A. Ahrendt (Department of Surgery, University of Pittsburgh Medical Center Shaydyside Hospital, Pittsburgh, USA); E. Akaishi (Department of Surgical Oncology, Centro de Oncologia Hospital Sirio Libanes, Sao Paolo, Brazil); S. H. Baik (Department of Surgery, Gangnam Severance Hospital - Yonsei University College of Medicine, Seoul, Korea); D. Baratti (Department of Gastrointestinal Surgery, San Raffaele Scientific Institute, Milan, Italy); A. Bhatt (Department of Surgical Oncology, Fortis Hospitals Limited, Bangalore, India); I. De Hingh (Department of Surgery, Catharina Ziekenhuis, Eindhoven, Netherlands); M. De Simone (Department of Surgical Oncology, Candiolo Cancer Institute - FPO, IRCCS, Turin, Italy); P. Dubé (Department of Surgery, University of Montreal, Montreal, Canada); R. P. Edwards (Department of Surgery, University of Pittsburgh Medical Center Shaydyside Hospital, Pittsburgh, USA); J. Franko (Department of Surgical Oncology, Mercy Medical Center, Baltimore, USA); L. Gonzalez-Bayon (Department of Surgical Oncology, Hospital Gregorio Marañón, Madrid, Spain); V. Gushchin (Department of Surgical Oncology, Mercy Medical Center, Baltimore, USA); M. P. Holtzman (Department of Surgery, University of Pittsburgh Medical Center Shaydyside Hospital, Pittsburgh, USA); M-C. Hsieh (Department of General Surgery, Wan-Fang Hospital, Taipei, Taiwan); D. Kecmanovic (Department of Surgery, First Surgical Clinic, Clinical Center of Serbia, Belgrade, Serbia); K. W. Lee (Department of Surgery, University of Pittsburgh Medical Center Shaydyside Hospital, Pittsburgh, USA); K. Lehmann (Department of Surgery and Transplantation, University Hospital of Zurich, Zurich, Switzerland); Y. Liu (NPO to Support Peritoneal Surface Malignancy Treatment, Kyoto, Japan); S. Mehta (Division of Peritoneal Surface Oncology, Saifee Hospital, Mumbai, India); D. L. Morris (Department of Surgery, University of New South Wales, Sydney, Australia); S. O'Dwyer (Department of Colorectal Surgery, Christie Cancer Center, Manchester, United Kingdom); E. Orsenigo (Department of Gastrointestinal Surgery, San Raffaele Scientific Institute, Milan, Italy); P. K. Pande (Department of Surgical Oncology, BLK Superspeciality Hospital, New Delhi, India); E. J. Park (Department of Surgery, Gangnam Severance Hospital - Yonsei University College of Medicine, Seoul, Korea); J. F. Pingpank (Department of Surgery, University of Pittsburgh Medical Center Shaydyside Hospital, Pittsburgh, USA); P. Piso (Department of Surgery, University of Regensburg, Regensburg, Germany); F. Rajan (Department of Surgical Oncology, Kovai Medical Centre, Coimbatore, India); B. Rau (Department of Surgical Oncology, Charite Campus Mitte University of Berlin, Berlin, Germany); A. Sardi (Department of Surgical Oncology, Mercy Medical Center Baltimore, USA); L. Sideris (Department of Surgery, University of Montreal, Montreal, Canada); A. Sommariva (Melanoma and Sarcoma Unit, Istituto Oncologico Veneto, Padua, Italy); J. Spiliotis (First Department of Surgical Oncology, Metaxa Cancer Memorial Hospital, Piraeus, Greece); A. A. K. Tentes (Department of Surgery, Metropolitan Hospital, Athens, Greece); M. Teo (Department of Surgical Oncology, National Cancer Centre Singapore, Singapore, Singapore); R. Yarema (Department of Oncology and Medical Radiology Danylo Halytsky Lviv National Medical University, Lviv, Ukraine); R. Younan (Department of Surgery, Centre Hospitalier de l’Université de Montréal, Montreal, Canada); S. S. Zaveri (Department of Surgical Oncology, Manipal Hospital, Bangalore, India); H. J. Zeh (Department of Surgery, University of Pittsburgh Medical Center, Shaydyside Hospital, Pittsburgh, USA).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest that are relevant to this article. O. Glehen declared financial activities outside this work in the form of consulting fees from GAMIDA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the PSOGI and BIG-RENAPE Working Group are listed in the Acknowledgements section.
Rights and permissions
About this article
Cite this article
Mercier, F., Passot, G., Bonnot, PE. et al. An International Registry of Peritoneal Carcinomatosis from Appendiceal Goblet Cell Carcinoma Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. World J Surg 46, 1336–1343 (2022). https://doi.org/10.1007/s00268-022-06498-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-022-06498-w