Abstract
Background
The progressive, systemic depletion of muscle mass is a poor prognostic factor for various types of cancers. However, the assessment of body composition for patients with esophagectomy remains unclear. Therefore, we evaluated the significance of the fat-free mass index (FFMI) and estimated the appropriate cutoff value.
Methods
We compiled clinicopathological characteristics of patients who underwent curative operation for esophageal cancer between October 2013 and March 2018 at Toranomon Hospital and reviewed them until December 2020. We analyzed the short- and long-term outcomes, compared to conventional nutritional factors, and calculated the area under the receiver operating characteristic (ROC) curve.
Results
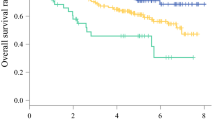
A total of 200 patients were eligible for inclusion. FFMI was ineffective in predicting postoperative complications, with no correlation with other nutritional biomarkers. Preoperative low FFMI led to poor overall survival (OS), and the lower cutoff values based on the time-dependent ROC analysis were 14.4 and 16.8 kg/m2 in women and men, respectively. Multivariate analysis for OS revealed that low FFMI (p = 0.010, HR 2.437, 95% CI 1.234–4.815) and clinical stage (p = 0.010, HR 4.781, 95% CI 1.447–15.796) were independent prognostic factors. The 3-year survival rates were 68.9% in low FFMI and 88.6% in normal FFMI.
Conclusions
The low FFMI was not predictive of postoperative complications but an independent prognostic factor in esophageal cancer with curative resection, having no correlation with other biomarkers. Our cutoff FFMI values could be useful in selecting the target for muscle improvement programs.





Similar content being viewed by others
References
Dodson S, Baracos VE, Jatoi A et al (2011) Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 62:265–279
Tamandl D, Paireder M, Asari R, Baltzer PA, Schoppmann SF, Ba-Ssalamah A (2016) Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol 26:1359–1367
Miyamoto Y, Baba Y, Sakamoto Y et al (2015) Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol 22:2663–2668
Cederholm T, Jensen GL, Correia MITD et al (2019) GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin Nutr 38:1–9
Cederholm T, Bosaeus I, Barazzoni R et al (2015) Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr 34:335–340
Roubenoff R, Baumgartner RN, Harris TB et al (1997) Application of bioelectrical impedance analysis to elderly populations. J Gerontol - Ser A Biol Sci Med Sci 52(3):M129–M136
Sergi G, De Rui M, Stubbs B, Veronese N, Manzato E (2017) Measurement of lean body mass using bioelectrical impedance analysis: a consideration of the pros and cons. Aging Clin Exp Res 29:591–597
Matsubara H, President F, Ando N et al (2017) Japanese classification of esophageal cancer, 11th edition: part I. Esophagus 14:1–36
Kitagawa Y, Uno T, Oyama T et al (2019) Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 1. Esophagus 16:1–24
Akiyama H, Miyazono H, Tsurumaru M et al (1978) Use of the stomach as an esophageal substitute. Ann Surg 188:606–610
Udagawa H, Akiyama H (2001) Surgical treatment of esophageal cancer: Tokyo experience of the three-field technique. Dis Esophagus 14:110–114
Udagawa H, Ueno M, Kinoshita Y (2009) Rationale for video-assisted radical esophagectomy. Gen Thorac Cardiovasc Surg 57:127–131
Udagawa H, Ueno M, Shinohara H et al (2012) The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 106(6):742–747
Udagawa H, Ueno M, Shinohara H et al (2014) Should lymph nodes along the thoracic duct be dissected routinely in radical esophagectomy? Esophagus 11:204–210
Xue Y, Zhou X, Xue L, Zhou R, Luo J (2019) The role of pretreatment prognostic nutritional index in esophageal cancer: a meta-analysis. J Cell Physiol 234:19655–19662
Kinoshita A, Onoda H, Imai N et al (2012) Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer 107:988–993
Ignacio De Ulíbarri J, González-Madroño A, De Villar NGP et al (2005) CONUT A tool for controlling nutritional status first validation in a hospital population. Nutr Hosp 20:38–45
Kuroda D, Sawayama H, Kurashige J et al (2018) Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer 21:204–212
Lee DH, Keum N, Hu FB et al (2017) Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Br J Nutr 118:858–866
Heagerty PJ, Lumley T, Pepe MS (2000) Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 56:337–344
Kudou K, Saeki H, Nakashima Y et al (2019) Postoperative development of sarcopenia is a strong predictor of a poor prognosis in patients with adenocarcinoma of the esophagogastric junction and upper gastric cancer. Am J Surg 217:757–763
Harada K, Ida S, Baba Y et al (2016) Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus 29:627–633
Ida S, Watanabe M, Yoshida N et al (2015) Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann Surg Oncol 22:4432–4437
Prado CMM, Lieff JR, Mccargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Demling RH (2009) Nutrition, anabolism, and the wound healing process: an overview. Eplasty 9:e9
Aoyama T, Kawabe T, Fujikawa H et al (2015) Loss of lean body mass as an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol 22:2560–2566
Nakashima Y, Saeki H, Nakanishi R et al (2018) Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg 267:1100–1104
Muscaritoli M, Anker SD, Argiles J et al (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by special interest groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr 29:154–159
Tan LJ, Liu SL, Lei SF et al (2012) Molecular genetic studies of gene identification for sarcopenia. Hum Genet 131:1–31
Prado CMM, Baracos VE, McCargar LJ et al (2007) Body composition as an independent determinant of 5-fluorouracil based chemotherapy toxicity. Clin Cancer Res 13:3264–3268
Schutz Y, Kyle UUG, Pichard C (2002) Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord 26:953–960
Kyle UG, Schutz Y, Dupertuis YM, Pichard C (2003) Body composition interpretation: contributions of the fat-free mass index and the body fat mass index. Nutrition 19:597–604
Beck FK, Rosenthal TC, York N (2002) Prealbumin: a marker for nutritional evaluation. Am Fam Phys 65:1575–1578
Bharadwaj S, Ginoya S, Tandon P et al (2016) Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep 4:272–280
Rossi AP, D’Introno A, Rubele S et al (2017) The Potential of β-Hydroxy-β-methylbutyrate as a new strategy for the management of sarcopenia and sarcopenic obesity. Drugs Aging 34:833–840
Koyama Y, Moro K, Nakano M et al (2017) Intravenous carnitine administration in addition to parenteral nutrition with lipid emulsion may decrease the inflammatory reaction in postoperative surgical patients. J Clin Med Res 9:831–837
Ohara M, Ogawa K, Suda G et al (2018) L-carnitine suppresses loss of skeletal muscle mass in patients with liver cirrhosis. Hepatol Commun 2:910–922
Tamaki M, Miyashita K, Hagiwara A et al (2017) Ghrelin treatment improves physical decline in sarcopenia model mice through muscular enhancement and mitochondrial activation. Endocr J 64:S47–S51
Yanagita I, Fujihara Y, Kitajima Y et al (2019) A high serum cortisol/DHEA-S ratio is a risk factor for sarcopenia in elderly diabetic patients. J Endocr Soc 3:801–813
Yamamoto K, Nagatsuma Y, Fukuda Y et al (2017) Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer 20:913–918
Acknowledgements
We thank Prof. Hideo Yasunaga, Department of Clinical Epidemiology and Health Economics, The University of Tokyo, for his helpful advice on statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest, and this work is not supported by any financial assistance funds.
Ethical approval
This study was approved by the research ethics committee of Toranomon Hospital (no. 2087).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yago, A., Ohkura, Y., Ueno, M. et al. Identification of Preoperative Fat-Free Mass Index for the Prognosis of Curatively Resected Esophageal Cancer. World J Surg 46, 845–854 (2022). https://doi.org/10.1007/s00268-021-06435-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-06435-3