Abstract
Background
Development of laparoscopic gastrectomy and the Enhanced Recovery After Surgery (ERAS) protocol enable early discharge to home of patients with gastric cancer (GC). However, a significant proportion of patients are still discharged to inpatient facilities after surgery. We aimed to identify predictive factors of non-home discharge in patients with GC who undergo gastrectomy.
Methods
We enrolled 517 patients with histopathologically confirmed diagnosis of GC who underwent gastrectomy.
Results
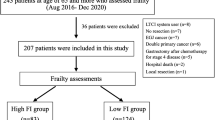
The number of patients with non-home discharge was 23 (4.4%), and non-home discharge was only observed in patients with GC aged ≥65 years. Patients were divided into the mFIHigh (≥0.272) and mFILow (<0.272) groups according to the cut-off value determined by ROC analysis. The mFIHigh classification was significantly more frequent in patients aged ≥75 years, who underwent either total or proximal partial gastrectomy, who underwent limited lymph node dissection, and with non-home discharge than in patients aged <75 years (p = 0.0002), those who underwent distal partial gastrectomy (p = 0.032), those who underwent standard lymph node dissection (p = 0.036), and those without non-home discharge (p = 0.0071). Multivariate analysis revealed mFI as an independent predictive indicator of non-home discharge, along with postoperative complications and surgical approach, in patients with GC aged ≥65 years. The frequency of patients with non-home discharge was significantly associated with the number of these three predictive factors in GC patients aged ≥65 years (p < 0.0001).
Conclusions
The combination of mFI, postoperative complications, and surgical approach is useful for predicting non-home discharge in patients aged ≥65 years who underwent gastrectomy for GC.





Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Japanese Gastric Cancer Association (2018) Gastric cancer treatment guidelines 2018. KANEHARA & CO., LTD, Tokyo
Sasako M, Sano T, Yamamoto S et al (2008) D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. New Engl J Med 359:453–462
Japanese Gastric Cancer Association (2017) Japanese Classification of Gastric Carcinoma. KANEHARA & CO., LTD, Tokyo
Velanovich V, Antoine H, Swartz A et al (2013) Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res 183:104–110
Onodera T, Goseki N, Kosaki G (1984) Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai zasshi 85:1001–1005
Kitamura K, Yamaguchi T, Taniguchi H et al (1996) Clinicopathological characteristics of gastric cancer in the elderly. Br J Cancer 73:798–802
Partridge JS, Harari D, Dhesi JK (2012) Frailty in the older surgical patient: a review. Age Ageing 41:142–147
Buettner S, Wagner D, Kim Y et al (2016) Inclusion of Sarcopenia outperforms the modified frailty index in predicting 1-year mortality among 1326 patients undergoing gastrointestinal surgery for a malignant indication. J Am Coll Surg 222:397–407.e392
Wagner D, DeMarco MM, Amini N et al (2016) Role of frailty and Sarcopenia in predicting outcomes among patients undergoing gastrointestinal surgery. World J Gastrointest Surg 8:27–40
Wagner D, Buttner S, Kim Y et al (2016) Clinical and morphometric parameters of frailty for prediction of mortality following hepatopancreaticobiliary surgery in the elderly. Br J Surg 103:e83–e92
Handforth C, Clegg A, Young C et al (2015) The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol Off J Eur Soc Med Oncol 26:1091–1101
Lee DH, Buth KJ, Martin BJ et al (2010) Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 121:973–978
Makary MA, Segev DL, Pronovost PJ et al (2010) Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 210:901–908
Joseph B, Pandit V, Rhee P et al (2014) Predicting hospital discharge disposition in geriatric trauma patients: Is frailty the answer? J Trauma Acute Care Surg 76:196–200
Courtney-Brooks M, Tellawi AR, Scalici J et al (2012) Frailty: an outcome predictor for elderly gynecologic oncology patients. Gynecol Oncol 126:20–24
Karam J, Tsiouris A, Shepard A et al (2013) Simplified frailty index to predict adverse outcomes and mortality in vascular surgery patients. Ann Vasc Surg 27:904–908
Partridge JS, Harari D, Martin FC et al (2014) The impact of pre-operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: a systematic review. Anaesthesia 69(Suppl 1):8–16
Bilimoria KY, Liu Y, Paruch JL et al (2013) Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 217:833–842.e831-833
Mogal HD, Fino N, Clark C et al (2016) Comparison of observed to predicted outcomes using the ACS NSQIP risk calculator in patients undergoing pancreaticoduodenectomy. J Surg Oncol 114:157–162
Copeland GP, Jones D, Walters M (1991) POSSUM: a scoring system for surgical audit. Br J Surg 78:355–360
Haga Y, Ikei S, Ogawa M (1999) Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today 29:219–225
Louwers L, Schnickel G, Rubinfeld I (2016) Use of a simplified frailty index to predict Clavien 4 complications and mortality after hepatectomy: analysis of the National Surgical Quality Improvement Project database. Am J Surg 211:1071–1076
Obeid NM, Azuh O, Reddy S et al (2012) Predictors of critical care-related complications in colectomy patients using the National Surgical Quality Improvement Program: exploring frailty and aggressive laparoscopic approaches. J Trauma Acute Care Surg 72:878–883
Adams P, Ghanem T, Stachler R et al (2013) Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg 139:783–789
Kitano S, Iso Y, Moriyama M et al (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Sakuramoto S, Kikuchi S, Kuroyama S et al (2006) Laparoscopy-assisted distal gastrectomy for early gastric cancer: experience with 111 consecutive patients. Surg Endosc 20:55–60
Mochiki E, Kamiyama Y, Aihara R et al (2005) Laparoscopic assisted distal gastrectomy for early gastric cancer: five years’ experience. Surgery 137:317–322
Kim MC, Jung GJ, Kim HH (2007) Morbidity and mortality of laparoscopy-assisted gastrectomy with extraperigastric lymph node dissection for gastric cancer. Dig Dis Sci 52:543–548
Park YK, Yoon HM, Kim YW et al (2018) Laparoscopy-assisted versus open D2 distal gastrectomy for advanced gastric cancer: results from a randomized phase II multicenter clinical trial (COACT 1001). Ann Surg 267:638–645
Acknowledgements
We would like to thank Prof. Yoichi Kurosawa for his helpful comments on statics and Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicting financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Osaki, T., Saito, H., Shimizu, S. et al. Modified Frailty Index is Useful in Predicting Non-home Discharge in Elderly Patients with Gastric Cancer Who Undergo Gastrectomy. World J Surg 44, 3837–3844 (2020). https://doi.org/10.1007/s00268-020-05691-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-020-05691-z