Abstract
Background
Molecular diagnostics can allow some patients with indeterminate thyroid nodule cytopathology to avoid diagnostic hemithyroidectomy; however, the testing is costly. We hypothesized that molecular testing with the intention of preventing unnecessary diagnostic hemithyroidectomy would be cost-effective if this test was applied selectively based on sonographic risk of malignancy.
Methods
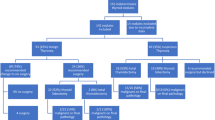
A Markov model was constructed depicting a 40-year-old patient with a cytologically indeterminate thyroid nodule. Molecular testing of fine needle aspiration material was compared to a strategy of immediate diagnostic hemithyroidectomy. Data from a single tertiary-referral health system were reviewed to estimate the outcomes of molecular testing of indeterminate nodules stratified by the American Thyroid Association sonographic classification system. Other outcome probabilities and their utilities were derived from literature review. Costs were estimated with Medicare reimbursement data. A $100,000/QALY threshold for cost-effectiveness was applied. Sensitivity analysis was employed to examine uncertainty in the model’s assumptions.
Results
Of 123 patients who underwent molecular testing for indeterminate cytology, 12 (9.8%) were classified as high sonographic suspicion, 49 (40%) were intermediate suspicion, and 62 (50%) were low or very low suspicion. Molecular testing was only cost-effective when the pretest probability of a negative test was greater than 31%. The model was most sensitive to the cost of molecular testing and the quality adjustment factor for hypothyroidism.
Conclusions
In hypothetical modeling, molecular testing is only cost-effective for cytologically indeterminate thyroid nodules with sonographic features that are intermediate or low suspicion for malignancy. In nodules with high sonographic suspicion, molecular testing is rarely negative and appears to add minimal value.




Similar content being viewed by others
References
Ali SZ, Cibas ES (eds) (2018) The Bethesda system for reporting thyroid cytopathology: definitions, criteria, and explanatory notes, 2nd edn. Springer, Cham
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE et al (2016) 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26(1):1–133
Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J et al (2012) Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. https://doi.org/10.1056/nejmoa1203208
Labourier E, Shifrin A, Busseniers AE, Lupo MA, Manganelli ML, Andruss B et al (2015) Molecular testing for miRNA, mRNA, and DNA on fine-needle aspiration improves the preoperative diagnosis of thyroid nodules with indeterminate cytology. J Clin Endocrinol Metab 100(7):2743–2750. https://doi.org/10.1210/jc.2015-1158
Lithwick-Yanai G, Dromi N, Shtabsky A, Morgenstern S, Strenov Y, Feinmesser M et al (2017) Multicentre validation of a microRNA-based assay for diagnosing indeterminate thyroid nodules utilising fine needle aspirate smears. J Clin Pathol 70(6):500–507. https://doi.org/10.1136/jclinpath-2016-204089
Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris RL et al (2014) Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer 120(23):3627–3634. https://doi.org/10.1002/cncr.29038
Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL et al (2011) Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab 96(11):3390–3397. https://doi.org/10.1210/jc.2011-1469
Patel KN, Angell TE, Babiarz J, Barth NM, Blevins T, Duh QY et al (2018) Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules. JAMA Surg 153(9):817–824. https://doi.org/10.1001/jamasurg.2018.1153
Clinical Laboratory Fee Schedule Public Use File [Internet] (2018) Centers for Medicare and Medicaid Services. c2018 – [cited 2019 May 21]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html
Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA et al (2017) ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol 14(5):587–595
Kandil E, Krishnan B, Noureldine SI, Yao L, Tufano RP (2013) Hemithyroidectomy: a meta-analysis of postoperative need for hormone replacement and complications. ORL 75(1):6–17
Verloop H, Louwerens M, Schoones JW, Kievit J, Smit JW, Dekkers OM (2012) Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab 97(7):2243–2255
Overview of the Medicare Physician Fee Schedule Search 2018 [cited 2019 May 16]. Available from: https://www.cms.gov/apps/physician-fee-schedule/overview.aspx
Zanocco K, Elaraj D, Sturgeon C (2013) Routine prophylactic central neck dissection for low-risk papillary thyroid cancer: a cost-effectiveness analysis. Surgery 154(6):1148–1155 discussion 54-5
Consumer Price Index - Medical Care 2018 [cited 2019 May 14]. Available from: http://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths
Muennig P (2008) Cost-effectiveness analyses in health: a practical approach, vol xvi, 2nd edn. Jossey-Bass, San Francisco, p 266
Sejean K, Calmus S, Durand-Zaleski I, Bonnichon P, Thomopoulos P, Cormier C et al (2005) Surgery versus medical follow-up in patients with asymptomatic primary hyperparathyroidism: a decision analysis. Eur J Endocrinol 153(6):915–927
Neumann PJ, Cohen JT, Weinstein MC (2014) Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 371(9):796–797. https://doi.org/10.1056/NEJMp1405158
Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J et al (2012) Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med 367(8):705–715. https://doi.org/10.1056/NEJMoa1203208
Li H, Robinson KA, Anton B, Saldanha IJ, Ladenson PW (2011) Cost-Effectiveness of a novel molecular test for cytologically indeterminate thyroid nodules. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2011-0459
Balentine CJ, Vanness DJ, Schneider DF (2018) Cost-effectiveness of lobectomy versus genetic testing (Afirma®) for indeterminate thyroid nodules: considering the costs of surveillance. Surgery 163(1):88–96
Labourier E (2016) Utility and cost-effectiveness of molecular testing in thyroid nodules with indeterminate cytology. Clin Endocrinol 85(4):624–631
Lee L, How J, Tabah RJ, Mitmaker EJ (2014) Cost-effectiveness of molecular testing for thyroid nodules with atypia of undetermined significance cytology. J Clin Endocrinol Metab 99(8):2674–2682
Wu JX, Lam R, Levin M, Rao J, Sullivan PS, Yeh MW (2016) Effect of malignancy rates on cost-effectiveness of routine gene expression classifier testing for indeterminate thyroid nodules. Surgery 159(1):118–129
Yip L, Farris C, Kabaker AS, Hodak SP, Nikiforova MN, McCoy KL et al (2012) Cost impact of molecular testing for indeterminate thyroid nodule fine-needle aspiration biopsies. J Clin Endocrinol Metab 97(6):1905–1912
Harrell RM, Eyerly-Webb SA, Golding AC, Edwards CM, Bimston DN (2018) Statistical comparison of Afirma GSC and Afirma GEC outcomes in a community endocrine surgical practice: early findings. Endocr Pract 25(2):161–164
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
IRB approval
The collection of human participant data for this study was approved by the UCLA institutional review board (UCLA IRB#: 18-000441, ATA Sonographic Classification and Molecular Test Results of Thyroid Nodules).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zanocco, K.A., Wang, M.M., Yeh, M.W. et al. Selective use of Molecular Testing Based on Sonographic Features of Cytologically Indeterminate Thyroid Nodules: A Decision Analysis. World J Surg 44, 393–401 (2020). https://doi.org/10.1007/s00268-019-05177-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05177-7