Abstract
Background
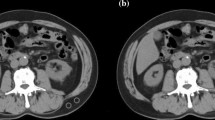
Skeletal muscle quality is a prognostic factor in various cancers. However, similar studies on curatively resected gastric cancer are lacking. We evaluated skeletal muscle quality using intramuscular adipose tissue content (IMAC) to clarify its impact on survival in patients with locally advanced gastric cancer.
Methods
We reviewed 370 patients who underwent curative resection for stage II/III gastric cancer. IMAC was calculated using preoperative computed tomography images. IMAC cutoff values were determined for each sex and were set at the 75th percentile. The patients were classified into normal and high IMAC groups according to the cutoff values. Clinicopathological factors and survival outcomes were compared between the two groups. Multivariate Cox regression analysis was used to identify independent prognostic factors for overall survival (OS) and cancer-specific survival (CSS).
Results
In all, 277 patients were classified into the normal IMAC group and 93 were classified into the high IMAC group. The patients in the high IMAC group were older, more obese, and had more comorbidities and poor Eastern Cooperative Oncology Group performance status than those in the normal IMAC group. Although no significant differences were observed in the pathological findings between the two groups, a high IMAC was significantly associated with poor OS and CSS. Multivariate analysis identified high IMAC as an independent prognostic factor for both OS and CSS (p = 0.046 and p = 0.035, respectively).
Conclusions
High IMAC was significantly associated with poor survival, suggesting that skeletal muscle quality has oncological implications in patients with locally advanced gastric cancer.



Similar content being viewed by others
References
Papenfuss WA, Kukar M, Oxenberg J et al (2014) Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol 21:3008–3014
Zhuang CL, Huang DD, Pang WY et al (2016) Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine 95:e3164
Kawamura T, Makuuchi R, Tokunaga M et al (2018) Long-term outcomes of gastric cancer patients with preoperative sarcopenia. Ann Surg Oncol 25:1625–1632
Huang DD, Chen XX, Chen XY et al (2016) Sarcopenia predicts 1-year mortality in elderly patients undergoing curative gastrectomy for gastric cancer: a prospective study. J Cancer Res Clin Oncol 142:2347–2356
Aoyama T, Sato T, Segami K et al (2016) Risk factors for the loss of lean body mass after gastrectomy for gastric cancer. Ann Surg Oncol 23:1963–1970
Sakurai K, Kubo N, Tamura T et al (2017) Adverse effects of low preoperative skeletal muscle mass in patients undergoing gastrectomy for gastric cancer. Ann Surg Oncol 24:2712–2719
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423
Marcus RL, Addison O, Kidde JP et al (2010) Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging 14:362–366
Goodpaster BH, Carlson CL, Visser M et al (1985) (2001) Attenuation of skeletal muscle and strength in the elderly: The health ABC study. J Appl Physiol 90:2157–2165
Ryall JG, Schertzer JD, Lynch GS (2008) Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 9:213–228
Hamaguchi Y, Kaido T, Okumura S et al (2015) Preoperative intramuscular adipose tissue content is a novel prognostic predictor after hepatectomy for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 22:475–485
Okumura S, Kaido T, Hamaguchi Y et al (2015) Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery 157:1088–1098
Okumura S, Kaido T, Hamaguchi Y et al (2016) Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery 159:821–833
Joglekar S, Asghar A, Mott SL et al (2015) Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol 111:771–775
Buettner S, Wagner D, Kim Y et al (2016) Inclusion of sarcopenia outperforms the modified frailty index in predicting 1-year mortality among 1326 patients undergoing gastrointestinal surgery for a malignant indication. J Am Coll Surg 222:397–407.e2
Wagner D, Buttner S, Kim Y et al (2016) Clinical and morphometric parameters of frailty for prediction of mortality following hepatopancreaticobiliary surgery in the elderly. Br J Surg 103:e83–92
Lu J, Zheng ZF, Li P et al (2018) A novel preoperative skeletal muscle measure as a predictor of postoperative complications, long-term survival and tumor recurrence for patients with gastric cancer after radical gastrectomy. Ann Surg Oncol 25:439–448
Kitajima Y, Eguchi Y, Ishibashi E et al (2010) Age-related fat deposition in multifidus muscle could be a marker for nonalcoholic fatty liver disease. J Gastroenterol 45:218–224
Kitajima Y, Hyogo H, Sumida Y et al (2013) Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J Gastroenterol Hepatol 28:1507–1514
Hamaguchi Y, Kaido T, Okumura S et al (2014) Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl 20:1413–1419
Hamaguchi Y, Kaido T, Okumura S et al (2017) Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation 101:565–574
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Nishikawa H, Shiraki M, Hiramatsu A et al (2016) Japan society of hepatology guidelines for sarcopenia in liver disease (1st Edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 46:951–963
Hamaguchi Y, Kaido T, Okumura S et al (2016) Muscle steatosis is an independent predictor of postoperative complications in patients with hepatocellular carcinoma. World J Surg 40:1959–1968. https://doi.org/10.1007/s00268-016-3504-3.
Zoico E, Corzato F, Bambace C et al (2013) Myosteatosis and myofibrosis: relationship with aging, inflammation and insulin resistance. Arch Gerontol Geriatr 57:411–416
Park J, Morley TS, Kim M et al (2014) Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 10:455–465
Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8:457–465
Dalamaga M (2013) Interplay of adipokines and myokines in cancer pathophysiology: emerging therapeutic implications. World J Exp Med 3:26–33
Hamaguchi Y, Kaido T, Okumura S et al (2016) Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 32:1200–1205
Chen LK, Liu LK, Woo J et al (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15:95–101
Carnevale V, Castriotta V, Piscitelli PA et al (2018) Assessment of skeletal muscle mass in older people: comparison between 2 anthropometry-based methods and dual-energy X-ray absorptiometry. J Am Med Dir Assoc 19:793–796
Maddocks M, Murton AJ, Wilcock A (2011) Improving muscle mass and function in cachexia: non-drug approaches. Curr Opin Support Palliat Care 4:361–364
Argiles JM, Busquets S, Lopez-Soriano FJ et al (2012) Are there any benefits of exercise training in cancer cachexia? J Cachexia Sarcopenia Muscle 2:73–76
Di Girolamo FG, Situlin R, Mazzucco S et al (2014) Omega-3 fatty acids and protein metabolism: enhancement of anabolic interventions for sarcopenia. Curr Opin Clin Nutr Metab Care 2:145–150
Dallmann R, Weyermann P, Anklin C et al (2011) The orally active melanocortin-4 receptor antagonist BL-6020/979: a promising candidate for the treatment of cancer cachexia. J Cachexia Sarcopenia Muscle 3:163–174
Grossmann M (2014) Myostatin inhibition: a new treatment for androgen deprivation-induced sarcopenia? J Clin Endocrinol Metab 10:3625–3628
Acknowledgements
The authors would like to thank Ryo Ashida for his helpful advice during this study.
Funding
The funding source was not involved in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Masanori Terashima has received personal fees from Taiho, Chugai, Ono, Bristol-Myers Squibb, Yakult, Takeda, Eli Lilly, Pfizer, and Daiichi Sankyo, outside the submitted work. All other authors declare no conflict of interest.
Human and animal rights
This study was approved by the institutional review board of Shizuoka Cancer Center (approval no. 29-J163-29-1-3).
Informed consent
Informed consent was not obtained from the patients. Instead, all patients were previously informed of an opt-out method.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Waki, Y., Irino, T., Makuuchi, R. et al. Impact of Preoperative Skeletal Muscle Quality Measurement on Long-Term Survival After Curative Gastrectomy for Locally Advanced Gastric Cancer. World J Surg 43, 3083–3093 (2019). https://doi.org/10.1007/s00268-019-05145-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05145-1