Abstract
Background
Several studies have examined controlling nutritional status (CONUT), which is one of the useful biomarkers for predicting patients’ prognosis following cancer treatment. The aim of this study was to evaluate the value of CONUT as a postoperative prognostic marker in patients with intrahepatic cholangiocarcinoma (ICC) following curative hepatectomy.
Methods
We retrospectively analyzed 71 patients who underwent curative hepatectomy for ICC between May 2002 and November 2016. Patients were divided into two groups according to their preoperative CONUT score (i.e., CONUT ≧ 2 or CONUT < 2).
Results
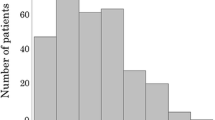
The number of patients assigned to the normal, mild, moderate, or severe malnutrition groups was 40, 28, two, and one, respectively. The high CONUT group (CONUT ≧ 2) consisted of 31 patients (43.7%) and had a poor prognosis with regard to overall survival (OS) (p = 0.0149). A high CONUT score is also identified as one of the independent predictors of poor prognosis in OS (hazard ratio 3.02; 95% confidence interval 1.4–6.8; p = 0.007). However, in the current study, a high CONUT score was not associated with postoperative complications (Clavien–Dindo classification ≧ III or more).
Conclusions
CONUT may be useful for the preoperative assessment of prognosis in patients with ICC who have undergone curative hepatectomy.

Similar content being viewed by others
References
Khan SA, Taylor-Robinson SD, Toledano MB et al (2002) Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol 37:806–813
Shaib YH, Davila JA, McGlynn K, El-Serag HB (2004) Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 40:472–477
Yoon H, Min JK, Lee JW et al (2011) Acquisition of chemoresistance in intrahepatic cholangiocarcinoma cells by activation of AKT and extracellular signal-regulated kinase (ERK)1/2. Biochem Biophys Res Commun 405:333–337
de Jong MC, Nathan H, Sotiropoulos GC et al (2011) Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 29:3140–3145
Ohtsuka M, Ito H, Kimura F et al (2002) Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg 89:1525–1531
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Lin G, Liu Y, Li S et al (2016) Elevated neutrophil-to-lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinoma. Oncotarget 7:50963–50971
Chen Q, Dai Z, Yin D et al (2015) Negative impact of preoperative platelet-lymphocyte ratio on outcome after hepatic resection for intrahepatic cholangiocarcinoma. Medicine (Baltimore) 94:e574
Okuno M, Ebata T, Yokoyama Y et al (2016) Evaluation of inflammation-based prognostic scores in patients undergoing hepatobiliary resection for perihilar cholangiocarcinoma. J Gastroenterol 51:153–161
Yoshida N, Harada K, Baba Y et al (2017) Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbecks Arch Surg 402:333–341
Yoshida N, Baba Y, Shigaki H et al (2016) Preoperative nutritional assessment by controlling nutritional status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg 40:1910–1917. doi:10.1007/s00268-016-3549-3
Zhang C, Wang H, Ning Z et al (2016) Prognostic nutritional index serves as a predictive marker of survival and associates with systemic inflammatory response in metastatic intrahepatic cholangiocarcinoma. Onco Targets Ther 9:6417–6423
Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG et al (2005) CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 20:38–45
Tokunaga R, Sakamoto Y, Nakagawa S et al (2017) CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int J Colorectal Dis 32:99–106
Yamashita Y, Taketomi A, Morita K et al (2008) The impact of surgical treatment and poor prognostic factors for patients with intrahepatic cholangiocarcinoma: retrospective analysis of 60 patients. Anticancer Res 28:2353–2359
Edge S, Byrd DR, Compton CC et al (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Yamashita YI, Wang H, Kurihara T et al (2016) Clinical significances of preoperative classification of intrahepatic cholangiocarcinoma: different characteristics of perihilar vs. peripheral ICC. Anticancer Res 36:6563–6569
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Pinato DJ, North BV, Sharma R (2012) A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer 106:1439–1445
Chan AW, Chan SL, Wong GL et al (2015) Prognostic nutritional index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol 22:4138–4148
Doussot A, Lim C, Gomez Gavara C et al (2016) Multicentre study of the impact of morbidity on long-term survival following hepatectomy for intrahepatic cholangiocarcinoma. Br J Surg 103:1887–1894
Miyata T, Yamashita YI, Yamao T et al (2017) Prognostic impacts of postoperative complications in patients with intrahepatic cholangiocarcinoma after curative operations. Int J Clin Oncol 22:526–532
Liu Y, Cao X (2016) Characteristics and significance of the pre-metastatic niche. Cancer Cell 30:668–681
McMillan DC, Watson WS, O’Gorman P et al (2001) Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 39:210–213
Halazun KJ, Hardy MA, Rana AA et al (2009) Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 250:141–151
Mano Y, Shirabe K, Yamashita Y et al (2013) Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg 258:301–305
Gomez D, Morris-Stiff G, Toogood GJ et al (2008) Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol 97:513–518
Okuyama H, Ichikawa Y, Sun Y et al (2007) Cancer and all-cause mortalities are lower in the higher total cholesterol groups among general populations. World Rev Nutr Diet 96:37–54
Jiang SS, Weng DS, Jiang L et al (2016) The clinical significance of preoperative serum cholesterol and high-density lipoprotein-cholesterol levels in hepatocellular carcinoma. J Cancer 7:626–632
Villa GR, Hulce JJ, Zanca C et al (2016) An LXR-cholesterol axis creates a metabolic co-dependency for brain cancers. Cancer Cell 30:683–693
Calleros L, Lasa M, Toro MJ, Chiloeches A (2006) Low cell cholesterol levels increase NFkappaB activity through a p38 MAPK-dependent mechanism. Cell Signal 18:2292–2301
Itatsu K, Sasaki M, Harada K et al (2009) Phosphorylation of extracellular signal-regulated kinase 1/2, p38 mitogen-activated protein kinase and nuclear translocation of nuclear factor-kappaB are involved in upregulation of matrix metalloproteinase-9 by tumour necrosis factor-alpha. Liver Int 29:291–298
Mon NN, Hasegawa H, Thant AA et al (2006) A role for focal adhesion kinase signaling in tumor necrosis factor-alpha-dependent matrix metalloproteinase-9 production in a cholangiocarcinoma cell line, CCKS1. Cancer Res 66:6778–6784
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Use of the clinical data in this study was approved by the human ethics review committee of the graduate school of medicine, Kumamoto University.
Electronic supplementary material
Below is the link to the electronic supplementary material.
268_2017_4214_MOESM2_ESM.tiff
Figure shows relapse-free survival and overall survival in patients with ICC after curative hepatectomy according to NLR (a, b), PLR (c, d), mGPS (e, f), and PNI (g, h). There is no significant difference between the two groups with respect to any of the biomarkers in this figure. (TIFF 3565 kb)
268_2017_4214_MOESM3_ESM.tiff
Figure shows the relation between CONUT score and NLR. CONUT score and NLR recognized a significant positive correlation (r = 0.494, p = 0.0017). (TIFF 16352 kb)
Rights and permissions
About this article
Cite this article
Miyata, T., Yamashita, Yi., Higashi, T. et al. The Prognostic Impact of Controlling Nutritional Status (CONUT) in Intrahepatic Cholangiocarcinoma Following Curative Hepatectomy: A Retrospective Single Institution Study. World J Surg 42, 1085–1091 (2018). https://doi.org/10.1007/s00268-017-4214-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-4214-1