Abstract
Background
Platinum resistance enhances DNA damage repair through nucleotide excision repair mechanisms involving the excision repair cross-complementing group 1 (ERCC1), X-ray cross-complementing group 1 (XRCC1), and excision repair cross-complementing group 2 (ERCC2). We evaluated the correlation between the expression of these three DNA repair genes and clinical outcomes in patients with rectal cancer receiving FOLFOX-based preoperative chemoradiotherapy (CRT).
Methods
Using immunohistochemistry, we examined the expression of ERCC1, ERCC2, and XRCC1 in pre-CRT cancer tissues from 86 patients with rectal cancer who had undergone curative resection and preoperative CRT with FOLFOX-4 to identify potential predictors of clinical outcomes.
Results
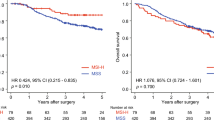
Following CRT, 57 and 29 patients were classified as responders (pathological tumor regression grade TRG 0 and TRG 1) and poor responders (TRG 2 and TRG 3), respectively. The multivariate analysis revealed that ERCC1 overexpression was correlated with a poor CRT response [p < 0.0001; odds ratio (OR), 9.397; 95% confidence interval (CI) 2.721–32.457]. Furthermore, a poor response to CRT (pathological TRG of 2–3) (p = 0.18; OR 5.685; 95% CI 1.349–23.954) and abnormal pre-CRT serum carcinoembryonic antigen levels (>5 ng/mL) (p = 0.03; OR 6.288; 95% CI 1.198–33.006) were independent predictors of postoperative relapse. By contrast, ERCC2 and XRCC1 expression did not play predictive roles in the analyzed patients.
Conclusions
ERCC1 overexpression is associated with a poor preoperative CRT response in patients with rectal cancer receiving FOLFOX-based preoperative CRT. ERCC1 is a potential biomarker for identifying patients who can benefit from customized treatment programs.



Similar content being viewed by others
References
Sauer R, Liersch T, Merkel S et al. (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30:1926–1933
Sauer R, Becker H, Hohenberger W et al. (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Bosset JF, Collette L, Calais G et al. (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123
Kalady MF, de Campos-Lobato LF, Stocchi L et al. (2009) Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg 250:582–589
Chen CF, Huang MY, Huang CJ et al. (2012) A observational study of the efficacy and safety of capecitabine versus bolus infusional 5-fluorouracil in pre-operative chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis 27:727–736
Ferrari L, Fichera A (2015) Neoadjuvant chemoradiation therapy and pathological complete response in rectal cancer. Gastroenterol Rep (Oxf) 3:277–288
Capirci C, Valentini V, Cionini L et al. (2008) Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 72:99–107
Garcia-Aguilar J, Hernandez de Anda E, Sirivongs P et al. (2003) A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum 46:298–304
Maas M, Nelemans PJ, Valentini V et al. (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11:835–844
de Gramont A, Figer A, Seymour M et al. (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Goldberg RM, Sargent DJ, Morton RF et al. (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23–30
Andre T, Boni C, Navarro M et al. (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27:3109–3116
Rodel C, Graeven U, Fietkau R et al. (2015) Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 16:979–989
Aschele C, Cionini L, Lonardi S et al. (2011) Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 29:2773–2780
Gerard JP, Azria D, Gourgou-Bourgade S et al. (2012) Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol 30:4558–4565
Quintieri L, Fantin M, Vizler C (2007) Identification of molecular determinants of tumor sensitivity and resistance to anticancer drugs. Adv Exp Med Biol 593:95–104
Ooyama A, Takechi T, Toda E et al. (2006) Gene expression analysis using human cancer xenografts to identify novel predictive marker genes for the efficacy of 5-fluorouracil-based drugs. Cancer Sci 97:510–522
Rajewsky MF, Muller R (2002) DNA repair and the cell cycle as targets in cancer therapy. In: Alison M (ed) The cancer handbook. Nature Publishing Group, London, pp 1507–1519
van de Vaart PJ, Belderbos J, de Jong D et al. (2000) DNA-adduct levels as a predictor of outcome for NSCLC patients receiving daily cisplatin and radiotherapy. Int J Cancer 89:160–166
Reed E (1998) Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat Rev 24:331–344
Amable L (2016) Cisplatin resistance and opportunities for precision medicine. Pharmacol Res 106:27–36
Altaha R, Liang X, Yu JJ et al. (2004) Excision repair cross complementing-group 1: gene expression and platinum resistance. Int J Mol Med 14:959–970
Weaver DA, Crawford EL, Warner KA et al. (2005) ABCC5, ERCC2, XPA and XRCC1 transcript abundance levels correlate with cisplatin chemoresistance in non-small cell lung cancer cell lines. Mol Cancer 4:18
Huang MY, Huang ML, Chen MJ et al. (2011) Multiple genetic polymorphisms in the prediction of clinical outcome of metastatic colorectal cancer patients treated with first-line FOLFOX-4 chemotherapy. Pharmacogenet Genomics 21:18–25
Metzger R, Leichman CG, Danenberg KD et al. (1998) ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol 16:309–316
Huang MY, Fang WY, Lee SC et al. (2008) ERCC2 2251A > C genetic polymorphism was highly correlated with early relapse in high-risk stage II and stage III colorectal cancer patients: a preliminary study. BMC Cancer 8:50
Huang MY, Tsai HL, Lin CH et al. (2013) Predictive value of ERCC1, ERCC2, and XRCC1 overexpression for stage III colorectal cancer patients receiving FOLFOX-4 adjuvant chemotherapy. J Surg Oncol 108:457–464
International Union Against Cancer (2002) TNM classification of malignant tumors, 6th edn. Wiley-Liss Inc, New York
Longo WE, Johnson FE (2002) The preoperative assessment and postoperative surveillance of patients with colon and rectal cancer. Surg Clin North Am 82:1091–1108
Huang CM, Huang MY, Tsai HL et al. (2016) An observational study of extending FOLFOX chemotherapy, lengthening the interval between radiotherapy and surgery, and enhancing pathological complete response rates in rectal cancer patients following preoperative chemoradiotherapy. Ther Adv Gastroenterol 9:702–712
Nagtegaal ID, Quirke P (2008) What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 26:303–312
Mace AG, Pai RK, Stocchi L et al. (2015) American Joint Committee on Cancer and College of American Pathologists regression grade: a new prognostic factor in rectal cancer. Dis Colon Rectum 58:32–44
Huang MY, Wang JY, Chang HJ et al. (2011) CDC25A, VAV1, TP73, BRCA1 and ZAP70 gene overexpression correlates with radiation response in colorectal cancer. Oncol Rep 25:1297–1306
Watanabe T, Kobunai T, Yamamoto Y et al. (2011) Gene expression signature and response to the use of leucovorin, fluorouracil and oxaliplatin in colorectal cancer patients. Clin Transl Oncol 13:419–425
McLeod HL, Sargent DJ, Marsh S et al. (2010) Pharmacogenetic predictors of adverse events and response to chemotherapy in metastatic colorectal cancer: results from North American Gastrointestinal Intergroup Trial N9741. J Clin Oncol 28:3227–3233
O’Connell MJ, Colangelo LH, Beart RW et al. (2014) Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol 32:1927–1934
Raymond E, Chaney SG, Taamma A et al. (1998) Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol 9:1053–1071
Graham MA, Lockwood GF, Greenslade D et al. (2000) Clinical pharmacokinetics of oxaliplatin: a critical review. Clin Cancer Res 6:1205–1218
Raymond E, Faivre S, Chaney S et al. (2002) Cellular and molecular pharmacology of oxaliplatin. Mol Cancer Ther 1:227–235
Gossage L, Madhusudan S (2007) Current status of excision repair cross complementing-group 1 (ERCC1) in cancer. Cancer Treat Rev 33:565–577
Xia Y, Hu C, Zhang M et al. (2007) Relationship between ERCC1 expression and cisplatin intervention in human lung adenocarcinoma cell lines. Zhongguo Fei Ai Za Zhi 10:362–365
Kim SH, Kwon HC, Oh SY et al. (2009) Prognostic value of ERCC1, thymidylate synthase, and glutathione S-transferase pi for 5-FU/oxaliplatin chemotherapy in advanced colorectal cancer. Am J Clin Oncol 32:38–43
Sung P, Bailly V, Weber C et al. (1993) Human xeroderma pigmentosum group D gene encodes a DNA helicase. Nature 365:852–855
Coin F, Marinoni JC, Rodolfo C et al. (1998) Mutations in the XPD helicase gene result in XP and TTD phenotypes, preventing interaction between XPD and the p44 subunit of TFIIH. Nat Genet 20:184–188
Lai JI, Tzeng CH, Chen PM et al. (2009) Very low prevalence of XPD K751Q polymorphism and its association with XPD expression and outcomes of FOLFOX-4 treatment in Asian patients with colorectal carcinoma. Cancer Sci 100:1261–1266
Park DJ, Stoehlmacher J, Zhang W et al. (2001) A Xeroderma pigmentosum group D gene polymorphism predicts clinical outcome to platinum-based chemotherapy in patients with advanced colorectal cancer. Cancer Res 61:8654–8658
Dabholkar M, Bostick-Bruton F, Weber C et al. (1992) ERCC1 and ERCC2 expression in malignant tissues from ovarian cancer patients. J Natl Cancer Inst 84:1512–1517
Ferry KV, Hamilton TC, Johnson SW (2000) Increased nucleotide excision repair in cisplatin-resistant ovarian cancer cells: role of ERCC1-XPF. Biochem Pharmacol 60:1305–1313
Fortini P, Pascucci B, Parlanti E et al. (2003) The base excision repair: mechanisms and its relevance for cancer susceptibility. Biochimie 85:1053–1071
Gangurde H, Chavan N, Mundada A et al. (2011) Biodegradable chitosan-based ambroxol hydrochloride microspheres: effect of cross-linking agents. J Young Pharm 3:9–14
Dai Q, Luo H, Li XP et al. (2015) XRCC1 and ERCC1 polymorphisms are related to susceptibility and survival of colorectal cancer in the Chinese population. Mutagenesis 30:441–449
Wang S, Wu X, Chen Y et al. (2012) Prognostic and predictive role of JWA and XRCC1 expressions in gastric cancer. Clin Cancer Res 18:2987–2996
Ang MK, Patel MR, Yin XY et al. (2011) High XRCC1 protein expression is associated with poorer survival in patients with head and neck squamous cell carcinoma. Clin Cancer Res 17:6542–6552
Caldecott KW (2003) XRCC1 and DNA strand break repair. DNA Repair (Amst) 2:955–969
Nix P, Greenman J, Stafford N et al. (2014) Expression of XRCC 1 and ERCC 1 proteins in radioresistant and radiosensitive laryngeal cancer. Cancer Therapy 2:47–53
Cubillo A, Rodriguez-Pascual J, Lopez-Rios F et al. (2016) Phase II trial of target-guided personalized chemotherapy in first-line metastatic colorectal cancer. Am J Clin Oncol 39:236–242
Yamada Y, Boku N, Nishina T et al. (2013) Impact of excision repair cross-complementing gene 1 (ERCC1) on the outcomes of patients with advanced gastric cancer: correlative study in Japan Clinical Oncology Group Trial JCOG9912. Ann Oncol 24:2560–2565
Zhong X, Li QQ, Reed E (2003) SU5416 sensitizes ovarian cancer cells to cisplatin through inhibition of nucleotide excision repair. Cell Mol Life Sci 60:794–802
Acknowledgements
This work was supported by Grants from the Excellence for Cancer Research Center Grant, funding by the Ministry of Science and Technology (MOST103-2314-B-037-010-MY3, MOST105-2325-B-037-001) and the Ministry of Health and Welfare (MOHW106-TDU-B-212-144007), Health and Welfare Surcharge of Tobacco Products, Taiwan, Republic of China, and Kaohsiung Medical University Hospital (KMUH102-2M69, KMUH104-4M51, KMUHS10505, KMUHS10518, KMUHS10522, KMUHGCRC2016002). The study is supported by Kaohsiung Medical University “Aim for the Top University Grant”, the Center for Biomarkers and Biotech Drugs, (KMU-TP105C01, KMU-TP105C02, KMU-TP105C11, KMU-TP106005, KMU-TP105A14, K MU-DK106005, SH000113 [Give2Asia]) and the Grant of Biosignature in Colorectal Cancers, Academia Sinica, Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, MY., Huang, JJ., Huang, CM. et al. Relationship Between Expression of Proteins ERCC1, ERCC2, and XRCC1 and Clinical Outcomes in Patients with Rectal Cancer Treated with FOLFOX-Based Preoperative Chemoradiotherapy. World J Surg 41, 2884–2897 (2017). https://doi.org/10.1007/s00268-017-4070-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-4070-z