Abstract
Background
The impact of lymph node (LN) dissection on long-term outcomes for patients with colorectal cancer (CRC) perforation remains unclear. We aim to investigate factors associated with poor prognosis and recurrence in patients with CRC, with special reference to cancer perforation and LN dissection.
Methods
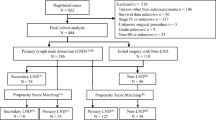
The subjects comprised 550 patients who underwent colorectal surgery for CRC at Stage II or III (TNM classification) between February 2006 and November 2013. Short- and long-term outcomes of patients with or without CRC perforation were evaluated. We also sought risk factors on poor prognosis, focusing on LN dissection in patients with CRC perforation.
Results
A total of 508 underwent surgery for CRC without perforation (the non-perforation group) and 39 for CRC with perforation (the perforation group). Both overall survival and recurrence-free survival rates were significantly lower in the perforation group than in the non-perforation group (overall survival, P = 0.009; recurrence-free survival, P < 0.001). The relapse rates at the peritoneum (P = 0.002), lung (P = 0.007) and LNs (P = 0.021) were significantly higher in the perforation group than in the non-perforation group. Multivariable Cox proportional hazards model revealed that CRC perforation (hazard ratio [HR] 2.55, 95 % confidential interval [CI] 1.16–4.98, P = 0.022), LN dissection <12 (HR 1.83, 95 % CI 1.07–3.13, P = 0.027), and Stage III (HR 1.79, 95 % CI 1.06–3.08, P = 0.031) were significant and independent risk factors for poor prognosis.
Conclusions
Conducting <12 LN dissections independently increased the risk of reduction in overall survival for patients with CRC perforation. Thus, radical LN dissections should be performed to improve patients’ survival rates, when patients’ general and surgical conditions allow.




Similar content being viewed by others
Abbreviations
- CRC:
-
Colorectal cancer
- CT:
-
Computed tomography
- LN:
-
Lymph node
- CI:
-
Confidential intervals
- HR:
-
Hazard ratio
References
GLOBOCAN 2012 website: http://globocan.iarc.fr (2012)
Benson AB 3rd, Bekaii-Saab T, Chan E et al (2013) Localized colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Cancer Netw JNCCN 11:519–528
Chen HS, Sheen-Chen SM (2000) Obstruction and perforation in colorectal adenocarcinoma: an analysis of prognosis and current trends Surgery 127:370–376
Carraro PG, Segala M, Orlotti C et al (1998) Outcome of large-bowel perforation in patients with colorectal cancer. Dis Colon Rectum 41:1421–1426
Mandava N, Kumar S, Pizzi WF et al (1996) Perforated colorectal carcinomas. Am J Surg 172:236–238
Steinberg SM, Barkin JS, Kaplan RS et al (1986) Prognostic indicators of colon tumors. Gastrointest Tumor Study Group Exp Cancer 57:1866–1870
Runkel NS, Schlag P, Schwarz V et al (1991) Outcome after emergency surgery for cancer of the large intestine. Br J Surg 78:183–188
Abdelrazeq AS, Scott N, Thorn C et al (2008) The impact of spontaneous tumour perforation on outcome following colon cancer surgery Colorectal disease. Off J Assoc Coloproctol G B Irel 10:775–780
McArdle CS, McMillan DC, Hole DJ (2006) The impact of blood loss, obstruction and perforation on survival in patients undergoing curative resection for colon cancer. Br J Surg 93:483–488
Khan S, Pawlak SE, Eggenberger JC et al (2001) Acute colonic perforation associated with colorectal cancer. Am Surg 67:261–264
Griffin MR, Bergstralh EJ, Coffey RJ et al (1987) Predictors of survival after curative resection of carcinoma of the colon and rectum. Cancer 60:2318–2324
Willett C, Tepper JE, Cohen A et al (1985) Obstructive and perforative colonic carcinoma: patterns of failure. J Clin Oncol Off J Am Soc Clin Oncol 3:379–384
Ho YH, Siu SK, Buttner P et al (2010) The effect of obstruction and perforation on colorectal cancer disease-free survival. World J Surg 34:1091–1101
Benson AB 3rd, Schrag D, Somerfield MR et al (2004) American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol Off J Am Soc Clin Oncol 22:3408–3419
Lee IK, Sung NY, Lee YS et al (2007) The survival rate and prognostic factors in 26 perforated colorectal cancer patients. Int J Colorectal Dis 22:467–473
Cheynel N, Cortet M, Lepage C et al (2009) Incidence, patterns of failure, and prognosis of perforated colorectal cancers in a well-defined population. Dis Colon Rectum 52:406–411
Wu Z-Y (2008) Risk factors for local recurrence of middle and lower rectal carcinoma after curative resection. World J Gastroenterol 14:4805
Harris GJ, Church JM, Senagore AJ et al (2002) Factors affecting local recurrence of colonic adenocarcinoma. Dis Colon Rectum 45:1029–1034
Sobin LH, Gospodarowicz MK, Wittekind C et al (2010) TNM classification of malignant tumours. Wiley-Blackwell
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Labianca R, Nordlinger B, Beretta GD et al (2013) Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol ESMO 24(Suppl 6):vi64–vi72
Gray RJ (1988) A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Glenn F, McSherry CK (1971) Obstruction and perforation in colo-rectal cancer. Ann Surg 173:983–992
Weiser MR, Landmann RG, Kattan MW et al (2008) Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol Off J Am Soc Clin Oncol 26:380–385
Quah HM, Chou JF, Gonen M et al (2008) Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum 51:503–507
Zaniboni A, Labianca R, Gruppo Italiano per lo Studio e la Cura dei Tumori del D (2004) Adjuvant therapy for stage II colon cancer: an elephant in the living room? Ann Oncol Off J Eur Soc Med Oncol ESMO 15:1310–1318
Merkel S, Wein A, Gunther K et al (2001) High-risk groups of patients with Stage II colon carcinoma. Cancer 92:1435–1443
Le Voyer TE, Sigurdson ER, Hanlon AL et al (2003) Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol Off J Am Soc Clin Oncol 21:2912–2919
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Kotaro Sugawara and Yoshikuni Kawaguchi have contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sugawara, K., Kawaguchi, Y., Nomura, Y. et al. Insufficient Lymph Node Sampling in Patients with Colorectal Cancer Perforation is Associated with an Adverse Oncological Outcome. World J Surg 41, 295–305 (2017). https://doi.org/10.1007/s00268-016-3667-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3667-y