Abstract
Background
Surgical audit is an essential task for the estimation of postoperative outcome and comparison of quality of care. Previous studies on surgical audits focused on short-term outcomes, such as postoperative mortality. We propose a surgical audit evaluating long-term outcome following colorectal cancer surgery. The predictive model for this audit is designated as ‘Estimation of Postoperative Overall Survival for Colorectal Cancer (EPOS-CC)’.
Methods
Thirty-one tumor-related and physiological variables were prospectively collected in 889 patients undergoing elective resection for colorectal cancer between April 2005 and April 2007 in 16 Japanese hospitals. Postoperative overall survival was assessed over a 5-years period. The EPOS-CC score was established by selecting significant variables in a uni- and multivariate analysis and allocating a risk-adjusted multiplication factor to each variable using Cox regression analysis. For validation, the EPOS-CC score was compared to the predictive power of UICC stage. Inter-hospital variability of the observed-to-estimated 5-years survival was assessed to estimate quality of care.
Results
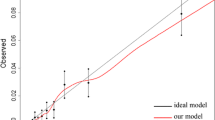
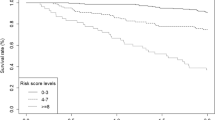
Among the 889 patients, 804 (90 %) completed the 5-years follow-up. Univariate analysis displayed a significant correlation with 5-years survival for 14 physiological and nine tumor-related variables (p < 0.005). Highly significant p-values below 0.0001 were found for age, ASA score, severe pulmonary disease, respiratory history, performance status, hypoalbuminemia, alteration of hemoglobin, serum sodium level, and for all histological variables except tumor location. Age, TNM stage, lymphatic invasion, performance status, and serum sodium level were independent variables in the multivariate analysis and were entered the EPOS-CC model for the prediction of survival. Risk-adjusted multiplication factors between 1.5 (distant metastasis) and 0.16 (serum sodium level) were accorded to the different variables. The predictive power of EPOS-CC was superior to the one of UICC stage; area under the curve 0.87, 95 % CI 0.85–0.90 for EPOS-CC, and 0.80, 0.76–0.83 for UICC stage, p < 0.001. Quality of care did not differ between hospitals.
Conclusions
The EPOS-CC score including the independent variables age, performance status, serum sodium level, TNM stage, and lymphatic invasion is superior to the UICC stage in the prediction of 5-years overall survival. This higher accuracy might be explained by the inclusion of physiological factors, thus also taking non-tumor-associated deaths into account. Furthermore, EPOS-CC score may compare quality of care among different institutions. Future studies are necessary to further evaluate this score and help improving the prediction of long-term survival following colorectal cancer surgery.



Similar content being viewed by others
References
Copeland GP, Jones D, Walters M (1991) POSSUM. A scoring system for surgical audit. Br J Surg 78:355–360
Khuri SF, Daley J, Henderson W et al (1998) The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 228:491–507
Fink AS, Campbell DA, Mentzer RM et al (2002) The National Surgical Quality Improvement Program in non-VA hospitals: initial demonstration of feasibility. Ann Surg 236:344–353
Khuri SF, Henderson WG, Daley J et al (2008) Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the patient safety in surgery study. Ann Surg 248:329–336
Haga Y, Ikei S, Ogawa M (1999) Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following GI surgery. Surg Today 29:219–225
Haga Y, Wada Y, Takeuchi H et al (2002) Estimation of surgical costs using a prediction scoring system estimation of physiologic ability and surgical stress. Arch Surg 137:481–485
Haga Y, Wada Y, Takeuchi H et al (2004) Estimation of physiologic ability and surgical stress (E-PASS) for a surgical audit in elective digestive surgery. Surgery 135:586–594
Haga Y, Ikejiri K, Wada Y et al (2011) A multicenter prospective study of surgical audit systems. Ann Surg 253:194–201
Haga Y, Wada Y, Ikenaga M et al (2011) Evaluation of modified Estimation of Physiologic Ability and Surgical Stress (mE-PSS) in colorectal carcinoma surgery. Dis Colon Rectum 54:1293–1300
Haga Y, Wada Y, Takeuchi H et al (2012) Evaluation of modified estimation of physiologic ability and surgical stress in gastric carcinoma surgery. Gastric Cancer 15:7–14
Haga Y, Ikejiri K, Takeuchi H et al (2012) Value of general surgical risk models for predicting postoperative liver failure and mortality following liver surgery. J Surg Oncol 106:898–904
Haga Y, Wada Y, Takeuchi H et al (2014) Evaluation of modified estimation of physiologic ability and surgical stress in patients undergoing surgery for choledochocystolithiasis. World J Surg 38:1177–1183. doi:10.1007/s00268-013-2383-0
Haga Y, Wada Y, Saitoh T et al (2014) Value of general surgical risk models for predicting postoperative morbidity and mortality in pancreatic resections for pancreatobiliary carcinomas. J Hepatobiliary Pancreat Sci. 21:599–606. doi:10.1002/jhbp.105
Japanese Society for Cancer of the Colon and Rectum (2005) JSCCR guidelines 2005 for the treatment of colorectal cancer. Kanehara & Co.Ltd, Tokyo
Nakamura T, Watanabe M (2013) Lateral lymph node dissection for lower rectal cancer. World J Surg 37:1808–1813. doi:10.1007/s00268-013-2072-z
Japanese Society for Cancer of Colon and Rectum (2009) General rules for clinical and pathological studies on cancer of the colon, rectum and anus, 7th edn. Kanehara & Co.Ltd, Tokyo
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Brosens RP, Oomen JL, Glas AS et al (2006) POSSUM predicts decreased overall survival in curative resection for colorectal cancer. Dis Colon Rectum 49:825–832
Nanashima A, Abo T, Nonaka T et al (2011) Prognosis of patients with hepatocellular carcinoma after hepatic resection: are elderly patients suitable for surgery? J Surg Oncol 104:284–291
Amemiya T, Oda K, Ando M et al (2007) Activities of daily living and quality of life of elderly patients after elective surgery for gastric and colorectal cancers. Ann Surg 246:222–228
Rees M, Tekkis PP, Welsh FK et al (2008) Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 247:125–135
Parekh AK, Goodman RA, Gordon C et al (2011) Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep 126:460–471
Nitsche U, Späth C, Müller TC et al (2014) Colorectal cancer surgery remains effective with rising patient age. Int J Colorectal Dis 29:971–979
Carlisle J, Swart M, Dawe EJ, Chadwick M (2012) Factors associated with survival after resection of colorectal adenocarcinoma in 314 patients. Br J Anaesth 108:430–435
Lai CC, You JF, Yeh CY et al (2011) Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis 26:473–481
Weiser MR, Landmann RG, Kattan MW et al (2008) Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol 26:380–385
Chok KS, Law WL (2007) Prognostic factors affecting survival and recurrence of patients with pT1 and pT2 colorectal cancer. World J Surg 31:1485–1490. doi:10.1007/s00268-007-9089-0
Iida S, Hasegawa H, Okabayashi K et al (2012) Risk factors for postoperative recurrence in patients with pathologically T1 colorectal cancer. World J Surg 36:424–430. doi:10.1007/s00268-011-1378-y
Huh JW, Lee JH, Kim HR et al (2013) Prognostic significance of lymphovascular or perineural invasion in patients with locally advanced colorectal cancer. Am J Surg 206:758–763
Berger AC, Sigurdson ER, LeVoyer T et al (2005) Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol 23:8706–8712
Gleisner AL, Mogal H, Dodson R et al (2013) Nodal status, number of lymph nodes examined, and lymph node ratio: what defines prognosis after resection of colon adenocarcinoma? J Am Coll Surg 217:1090–1100
Wang LP, Wang HY, Cao R et al (2013) Proposal of a new classification for stage III colorectal cancer based on the number and ratio of metastatic lymph nodes. World J Surg 37:1094–1102. doi:10.1007/s00268-013-1940-x
Haga Y, Ikejiri K, Wada Y et al (2015) Preliminary study of surgical audit for overall survival following gastric cancer resection. Gastric Cancer 18:138–146. doi:10.1007/s10120-014-0343-5
Inoue Y, Toiyama Y, Tanaka K et al (2011) Oncology market research provides a feasible index for standardization of colorectal cancer chemotherapy. Jpn J Clin Oncol 41:1203–1208
Acknowledgments
This work was supported by a grant of NHO Multi-Center Clinical Research for Evidence-Based Medicine. The authors wish to thank all of the institutional investigators listed below for their unstinting exertions in collecting data. H. Naito, M.D., NHO Hokkaido Cancer Center; M. Oohara, M.D., NHO Hokkaido Cardiovascular Center; A. Nagase, M.D., NHO Dohoku Hospital; H. Tsunoda, M.D., NHO Kofu National Hospital; K. Kondo, M.D., NHO Nagoya Medical Center; O. Kimura, M.D., NHO Yonago Medical Center; H. Takeuchi, M.D., NHO Iwakuni Clinical Center; T. Nakata, M.D., NHO Nagasaki Medical Center; H. Matsuzaki, M.D., NHO Kumamoto Medical Center; Y. Ogura, M.D., NHO Minami Kyushu National Hospital.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haga, Y., Ikejiri, K., Wada, Y. et al. The EPOS-CC Score: An Integration of Independent, Tumor- and Patient-Associated Risk Factors to Predict 5-years Overall Survival Following Colorectal Cancer Surgery. World J Surg 39, 1567–1577 (2015). https://doi.org/10.1007/s00268-015-2962-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-2962-3