Abstract
Background
We have previously reported the molecular detection of peritoneal micrometastases in patients with gastric cancer by quantifying carcinoembryonic antigen (CEA) mRNA in the peritoneal washes. Patients with CEA mRNA exceeding a cutoff value have a significant risk for developing peritoneal carcinomatosis, but optimal treatment for this population remains unknown.
Methods
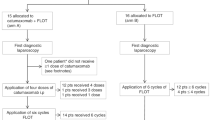
CEA mRNA (+) patients with gastric cancer were treated postoperatively with S-1 monotherapy. Overall survival, the primary endpoint of this phase II trial, was compared with the historical control, which is comprised of CEA mRNA (+) patients who were not given postoperative chemotherapy.
Results
A total of 32 patients with CEA mRNA (+) gastric cancer were enrolled. Twelve patients (37.5%) relapsed; ten showed peritoneal relapse. Three-year survival was similar between the study population and the historical control (67.3% vs. 67.1%, respectively).
Conclusions
S-1 monotherapy, which significantly reduced risk for recurrence in stage II/III gastric carcinoma in another phase III trial, seems not to be as effective in eradicating free cancer cells in the abdominal cavity.




Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Boku T, Nakane Y, Minoura T et al (1990) Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg 77:436–439
Kodera Y, Nakanishi H, Yamamura Y et al (1998) Prognostic value and clinical implications of disseminated cancer cells in the peritoneal cavity detected by reverse transcriptase-polymerase chain reaction and cytology. Int J Cancer 79:429–433
Ito S, Nakanishi H, Kodera Y et al (2005) Prospective validation of quantitative CEA mRNA detection in peritoneal washes in gastric carcinoma patients. Br J Cancer 93:986–992
Kodera Y, Nakanishi H, Ito S et al (2002) Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptase-polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg 235:499–506
Nakanishi H, Kodera Y, Torii A et al (1997) Detection of carcinoembryonic antigen-expressing free tumor cells in peritoneal washes from patients with gastric carcinoma by polymerase chain reaction. Jpn J Cancer Res 88:687–692
Nakanishi H, Kodera Y, Yamamura Y et al (2000) Rapid quantitative detection of carcinoembryonic antigen-expressing free tumor cells in the peritoneal cavity of gastric-cancer patients with real-time RT-PCR on the lightcycler. Int J Cancer 89:411–417
Chaudhuri TR, Mountz JM, Rogers BE et al (2001) Light-based imaging of green fluorescent protein-positive ovarian cancer xenografts during therapy. Gynecol Oncol 82:581–589
Kurebayashi J, Nukatsuka M, Fujioka A et al (1997) Postsurgical oral administration of uracil and tegafur inhibits progression of micrometastasis of human breast cancer cells in nude mice. Clin Cancer Res 3:653–659
Yokoyama H, Nakanishi H, Kodera Y et al (2006) Biological significance of isolated tumor cells and micrometastasis in lymph nodes evaluated using a green fluorescent protein-tagged human gastric cancer cell line. Clin Cancer Res 12:361–368
Hermans J, Bonenkamp JJ, Boon MC et al (1993) Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol 11:1441–1447
Shirasaka T, Shimamato Y, Ohshimo H et al (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7:548–557
Koizumi W, Kurihara M, Nakano S et al (2000) Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 58:191–197
Sakata Y, Ohtsu A, Horikoshi N et al (1998) Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34:1715–1720
Kinoshita T, Nashimoto A, Yamamura Y et al (2004) Feasibility study of adjuvant chemotherapy with S-1 (TS-1; tegafur, gimeracil, oteracil potassium) for gastric cancer. Gastric Cancer 7:104–109
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Nakanishi H, Kodera Y, Yamamura Y et al (1999) Molecular diagnostic detection of free cancer cells in the peritoneal cavity of patients with gastrointestinal and gynecologic malignancies. Cancer Chemother Pharmacol 43(Suppl):S32–S36
Wolfrum F, Vogel I, Fandrich F et al (2005) Detection and clinical implications of minimal residual disease in gastro-intestinal cancer. Langenbecks Arch Surg 390:430–441
Mori K, Suzuki T, Uozaki H et al (2007) Detection of minimal gastric cancer cells in peritoneal washings by focused microarray analysis with multiple markers: clinical implications. Ann Surg Oncol 14:1694–1702
Fujita Y, Terashima M, Hoshino Y et al (2006) Detection of cancer cells disseminated in bone marrow using real-time quantitative RT-PCR of CEA, CK19, and CK20 mRNA in patients with gastric cancer. Gastric Cancer 9:308–314
Mimori K, Fukagawa T, Kosaka Y et al (2008) Hematogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor-1. Clin Cancer Res 14:2609–2616
Kodera Y, Ito S, Mochizuki Y et al (2009) A phase II study of radical surgery followed by postoperative chemotherapy with S-1 for gastric carcinoma with free cancer cells in the peritoneal cavity (CCOG0301 study). Eur J Surg Oncol 35:1158–1163
Dalal KM, Woo Y, Kelly K et al (2008) Detection of micrometastases in peritoneal washings of gastric cancer patients by the reverse transcriptase polymerase chain reaction. Gastric Cancer 11:206–213
Kodera Y, Nakanishi H, Ito S et al (2006) Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: analysis of real time reverse transcriptase-polymerase chain reaction after 5 years of follow-up. J Am Coll Surg 202:231–236
Tsuburaya A, Sakamoto J, Morita S et al (2005) A randomized phase III trial of post-operative adjuvant oral fluoropyrimidine versus sequential paclitaxel/oral fluoropyrimidine; and UFT versus S1 for T3/T4 gastric carcinoma: the Stomach Cancer Adjuvant Multi-institutional Trial Group (Samit). Trial Jpn J Clin Oncol 35:672–675
Markman M (2003) Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol 4:277–283
Kang Y, Chang H, Zang D et al (2008) Postoperative adjuvant chemotherapy for grossly serosa-positive advanced gastric cancer: a randomized phase III trial of intraperitoneal cisplatin and early mitomycin-C plus long-term doxifluridine plus cisplatin (iceMFP) versus mitomycin-C plus short- term doxifluridine (Mf) (AMC 0101) (NCT00296322). J Clin Oncol 26(Suppl):abstr LBA4511
Kodera Y, Ito Y, Ito S et al (2007) Intraperitoneal paclitaxel: a possible impact of regional delivery for prevention of peritoneal carcinomatosis in patients with gastric carcinoma. Hepatogastroenterology 54:960–963
Ohashi N, Kodera Y, Nakanishi H et al (2005) Efficacy of intraperitoneal chemotherapy with paclitaxel targeting peritoneal micrometastasis as revealed by GFP-tagged human gastric cancer cell lines in nude mice. Int J Oncol 27:637–644
Acknowledgments
This study was supported in part by a grant from the Ministry of Health, Labor and Welfare, Japan and Ministry of Education, Science, Sports, Culture and Technology, Japan.
Conflict of interest
There are no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ito, S., Kodera, Y., Mochizuki, Y. et al. Phase II Clinical Trial of Postoperative S-1 Monotherapy for Gastric Cancer Patients with Free Intraperitoneal Cancer Cells Detected by Real-Time RT-PCR. World J Surg 34, 2083–2089 (2010). https://doi.org/10.1007/s00268-010-0573-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-010-0573-6