Abstract
Background
Controversy exists regarding the aggressiveness of initial therapy in childhood papillary thyroid cancer (PTC). Few studies with long-term outcome exist and second primary malignancies have rarely been analyzed.
Methods
We studied 215 PTC patients younger than 21 years old managed during 1940 through 2008. The patients were aged 3–20 year old (median age = 16 years); the median follow-up was 29 years. Recurrence and mortality details were taken from a computerized database.
Results
Median primary tumor size was 2.2 cm. Six percent had distant metastases at presentation, 5% had incomplete tumor resection, 86% had nodes removed at initial surgery, and 78% had nodal metastases. After complete surgical resection, PTC recurred in 32% by 40 years. At 20 years, the recurrence rates at local, regional, and distant sites were 7, 21, and 5%, respectively. During 1940–1969, local and regional recurrence rates after unilateral lobectomy (UL) were significantly (P < 0.001) higher than after bilateral lobar resection (BLR). During 1950–2008 radioiodine remnant ablation (RRA) was administered within 18 months to 32%; it did not diminish the 25-year regional recurrence rate of 16% seen after BLR alone (P = 0.86). Only two fatal events from PTC occurred at 28 and 30 years, for a cause-specific mortality at 40 years of only 2%. All-causes mortality rates did not exceed expectation through 20 years, but from 30 through 50 years, the number of deaths was significantly (P < 0.001) higher than predicted. Fifteen of 22 deaths (68%) resulted from nonthyroid malignancy.
Conclusion
Survival from childhood PTC should be expected, but later death from nonthyroid malignancy is disconcerting. Seventy-three percent of those who died from nonthyroid malignancy had received postoperative therapeutic irradiation.
Similar content being viewed by others
Introduction
Papillary thyroid cancer (PTC) is the most common thyroid follicular cell-derived malignancy. When it occurs in childhood and adolescence, PTC has, at initial presentation, more often metastasized to neck nodes and lungs than in adults [1, 2]. It is also more often recurrent in neck lymph nodes postoperatively. In a 1988 study from Mayo Clinic, distant metastases developed postoperatively by 30 years in 6.9% of children and 6.6% of adults with PTC [2]. However, we and others have previously demonstrated that children with PTC metastatic to lung have a much lower cause-specific mortality [3], when compared to adult patients with distant spread [2, 3]. The observations that PTC in childhood (1) is initially extensive, (2) more often displays distant spread, and (3) causes fewer deaths from PTC than comparably staged adult patients led Crile in 1959 [4] to recommend initial conservative surgical treatment.
By 1988 the spectrum of initial surgical management was illustrated by three consecutive papers [2, 5, 6] on childhood thyroid cancer presented at the Ninth Annual Meeting of the American Association of Endocrine Surgeons in Boston, Massachusetts. At Memorial Sloan-Kettering [5], lobectomy was the commonest procedure in 100 patients with differentiated thyroid cancer (DTC) treated during 1949–1986. Bilateral subtotal thyroidectomy or total thyroidectomy accounted for 74% of 58 Mayo PTC patients treated during 1946–1975 [2], and near-total thyroidectomy with or without neck dissection was the standard treatment in 48 children from Pisa [6] treated during 1971–1987. Use of radioiodine as part of initial therapy varied from 20% at Mayo to 23% at Memorial to 100% in Pisa. In spite of this varied spectrum of initial management policies, only two (1%) of the 206 patients died. One fatality at Mayo resulted at 30 postoperative years from a distant metastasis found only at autopsy [2], while the patient from Pisa died at 15 years after initial diagnosis from respiratory failure attributed to pulmonary fibrosis induced by the administration of a cumulative dose of 500 mCi of radioactive I-131 for pulmonary micrometastases [6].
In the 21 years since these papers have been published, the literature on PTC during childhood has hugely expanded as the 1986 Chernobyl accident has led to a dramatic incidence in pediatric PTC in inhabitants of the contaminated territories [7]. These post-Chernobyl patients have in general been treated rather aggressively [8, 9], and an “evidence-based review” from Toronto [10] has now concluded from the literature of the past 15 years that “all patients with DTC should undergo total or near-total thyroidectomy (TT/NTT) with selective lymph node dissection (when involved) as the initial treatment … and should undergo radioiodine remnant ablation (RRA), defined as the destruction of residually macroscopically normal thyroid tissue, within 4–6 weeks after the initial thyroidectomy (Grade B, Level III recommendation).”
In treating juveniles with PTC at our institution [11] we have, since 1940, evolved from performing unilateral lobectomy to doing routine near-total or total thyroidectomy and nodal dissection, while selectively using RRA [12]. In recent years, in which preoperative and postoperative ultrasound scanning has become routine, we are, as in our adult PTC practice, increasingly performing initial central compartment node procedures while progressively using less I-131 for postoperative remnant ablative purposes [13]. In this present study our aims were to define long-term outcome (recurrence and mortality) in a large cohort of PTC patients less than 21 years of age followed at our institution for up to 65 postoperative years and on average for almost 30 years. We were very interested in comparing patient outcome after unilateral lobectomy (UL) versus bilateral lobar resection (BLR). Also, we wished to determine the efficiency of RRA in reducing locoregional recurrence rates. Finally, we are concerned “that late complications of therapy may create significant morbidity” [14]. Specifically, we were interested to know whether patients surviving pediatric PTC might “develop possibly treatment-related lethal complications or second neoplasms” as has recently been suggested by our pediatric endocrine surgical colleagues from M.D. Anderson [14].
Methods
Patients and follow-up
The records of all patients younger than 21 years of age at initial PTC diagnosis, who underwent definitive primary surgical therapy at Mayo Clinic during a 68-year period between January 1, 1940 and December 31, 2008, were reviewed. This age range was deemed appropriate because of the indolent nature of this disease and the frequent delay in diagnosis. All relevant histologic slides were reexamined and reclassified according to current WHO criteria [15] by staff endocrine pathologists who had no knowledge of disease outcome. There were 218 patients who underwent primary surgical therapy at Mayo Clinic, had histologic reconfirmation of PTC, and were treated within 60 days of the initial diagnosis. The study protocol was approved by the Mayo Institutional Review Board. Two hundred fifteen (99%) of the 218 patients gave consent to participate in the follow-up study and are the subject of this report. Details of patients’ presentations, surgical and pathologic findings, and adjunctive treatments were obtained from the computerized Mayo Clinic Rochester Thyroid Cancer Database [11, 16], maintained since 1984 by one of us (IDH).
Death certificates were examined for the 22 patients (10%) who were known to have died. Information regarding the 193 living patients was obtained either by Mayo Clinic re-examination or through correspondence with the home physician, patient, or relatives; patients were followed up to June 2009. The median follow-up of the 215 patients was 28.7 years (range = 0.6–64.5 years), amounting to 5,838 patient-years of observation. Death was due to PTC in two (0.9%) cases. Studies of postoperative tumor recurrence involved 192 patients (89%) and did not include 12 patients (6%) who had distant metastases discovered within 30 days of the initial surgery and 11 patients (5%) who had incomplete tumor resection, with persistent gross residual disease [11, 13, 16].
Statistical analysis
Survival rates from the date of initial surgery until death (all causes or cause-specific) or tumor recurrence were estimated by the Kaplan-Meier method. Expected survival was based on 1975 Minnesota life-tables for patients of similar age and sex. Comparisons of risk characteristics and trends across the decades were performed using χ2 tests of proportion or Fisher’s exact test when necessary. The log-rank test was used to determine group differences in survival curves [17]. All tests were two-sided, with an α level of 0.05. All calculations were performed using SAS software (SAS Institute, Cary, NC) [18].
Results
Patient and tumor characteristics
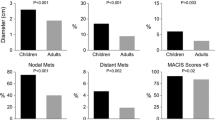
The median age at diagnosis of PTC was 16 years (range = 3–20 years). There were 152 female PTC patients (71%) and the female: male ratio was 2.4:1. In 36 patients (17%), there was a prior history of head or neck irradiation, which occurred during 1936 through 1957. The mean tumor size was 2.65 cm in maximum diameter (median = 2.20 cm; range = 0.1–9.5 cm). The histologic grade was 1 in 201 (93.5%) and 2 (of 4) in 14 (6.5%). DNA ploidy by flow cytometry was diploid in 51 (89%), tetraploid in 4 (7%), and aneuploid in 2 (4%) of 57 cases examined. Tumors were demonstrated to be multicentric in 29% of 190 examined specimens. Altogether, 39 (18%) of the primary tumors at presentation were locally invasive to extrathyroidal soft tissues and 12 (6%) had distant metastases found no later than 30 days after the date of the initial surgical procedure; 167 (78%) had metastatic involvement of regional lymph nodes at the time of initial surgery (60% had 4 or more metastatic nodes). By conventional risk-group classification, the 215 PTC patients were classified as “low-risk” in 90% by MACIS, 94% by AMES, and 98% by the AGES prognostic scoring system [19–21].
Initial thyroid surgery
Bilateral lobar resection (BLR) was performed in 188 (87%) juvenile PTC patients during the period of study. Overall, the most frequently performed primary surgical procedure was near-total thyroidectomy (NTT), which accounted for 96 (45%) of the initial operations. Total thyroidectomy (TT) was the second most popular operation and was performed in 82 cases (38%). Bilateral subtotal thyroidectomy (BST) was performed in only 10 cases (5%). Unilateral lobectomy (UL) was the primary surgical procedure in 25 cases (12%); lesser procedures were performed in the remaining 1%. In only 16 cases (7%) was the tumor excision incomplete, where the surgeon reported the persistence of gross residual disease at the conclusion of the initial neck operation. There were 192 patients (89%) who underwent complete surgical resection and had no distant metastases on initial examination or within 30 days of the primary operation.
Postoperative morbidity from initial thyroid surgery
Permanent unilateral recurrent laryngeal nerve (RLN) damage occurred in 12% after UL, in 0% after BST, in 6% after TT, and in 2% after NTT. Permanent hypoparathyroidism, requiring long-term calcium and vitamin D therapy, occurred in none of the 35 patients who had either a UL or a BST. However, permanent hypoparathyroidism resulted in 4% after NT and in 29% after TT. During 1950–1979, 22 of 43 patients who underwent TT (51%) developed permanent hypoparathyroidism, whereas since 1980, only 2 of 30 (5%) had such a postoperative consequence. In the 27 cases of juvenile PTC in which definitive initial thyroid surgery was used during 2000–2008, there have been no cases of permanent hypoparathyroidism and only one case (3%) of unilateral vocal cord paresis. This occurred in a 19-year-old female who had undergone a total thyroidectomy, central compartment node dissection, and bilateral modified dissection for a 5.5-cm bilateral multicentric tumor invading the trachea, esophagus, and left RLN and involving 20 of 24 resected central compartment and lateral (jugular chain) neck nodes.
Neck nodal surgery
One hundred eighty-five (86%) of the PTC patients underwent removal of neck nodes as part of the initial operation. Over the 68-year period studied, the most popular nodal surgical procedure, especially during the earlier decades, was “node-picking” from the central compartment. This was performed unilaterally in 51 (24%) and bilaterally in 13 (6%). Only 27 (13%) had a bilateral modified neck procedure as part of their initial surgical approach, while a total of 29 (13%) had a unilateral modified neck dissection. A combination of “picking” and lateral compartment dissection was performed in 25 (12%). Central compartment nodal dissection (CCND) routinely accompanied BLR in the period since 1995 and was performed in 40 cases (19%).
Postoperative radioiodine remnant ablation (RRA)
There were 192 patients (89%) whose tumor at presentation was confined to the neck and who underwent complete tumor resection, with no gross residual disease. These potentially curable patients were considered eligible for postoperative radioiodine adjunctive therapy. Postoperative thyroid remnant ablation with radioactive iodine was performed in 68 of these 192 PTC patients (35%). Fifty-four (79%) received only one dose, 9 (13%) had two doses, 4 (6%) had three doses, and only one received four doses. The cumulative dose varied between 29 and 400 mCi of I-131, with an average of 79 mCi. In 64 cases (94%) there was sufficient information available to assess the efficacy of ablative therapy, and 55 of these 64 (86%) were shown to have been ablated successfully, with the post-therapy scan showing no residual radioiodine-avid focus and uptake being no higher than background.
Cause-specific mortality
The complete cohort of 215 patients was followed for a median of 28.7 postoperative years (5,838 patient-years of observation). There were no deaths from PTC during the first 20 postoperative years, and none of the 12 who presented with distant metastases died from PTC. Death from PTC did occur, consequent to postoperative distant metastases, after 25 years in two patients (0.9%), both of whom had localized disease at presentation and had been treated with curative intent by bilateral lobar resection.
The first lethal case was a boy of 14 who had undergone thymic irradiation during the first year of life. He presented in 1950 with a node-positive 5-cm tumor and was treated initially with BST, jugular and mediastinal node dissection, and postoperative radium application. He developed lung and pleural metastases at 24 postoperative years. By 26 years, he had multiple osseous metastases and also had a 3-cm occipital metastasis. In 1978 he underwent a decompressive laminectomy for thoracic vertebral metastases but died of “tumor pneumonitis” at 5 postoperative days.
The second lethal case was even more unusual. She presented in 1948 with a neck nodal mass, which was biopsied and shown to be PTC. AT age 19 she underwent a NTT with selective nodal dissection for a node-positive 2.5-cm papillary tumor. The tumor was grossly resected and no adjunctive therapy was given other than thyroid hormone. At 6 postoperative months she had two contralateral neck nodes removed and was given soon thereafter an 8-day course of X-ray therapy. From 1949 until 1978, she was considered to be free of PTC. In 1978, she died suddenly and autopsy revealed the cause of death to have been bleeding into a 3.5-cm occipital brain metastasis. The patient also was found at autopsy to have a 1-cm temporal lobe metastasis and four metastatic nodules of PTC in her right lung.
To date, there have been no deaths from PTC in the 181 patients treated since 1951. Cause-specific survival rates were 100% at 20 years and 98% from 30 through 50 postoperative years, as illustrated in Fig. 1.
Postoperative recurrence
As in previous studies [11, 13, 22], postoperative recurrences were considered only for patients who had undergone a potentially curative operation as their initial treatment. Figure 2 shows the recurrence-free survival in the 192 patients with PTC who satisfied this criterion. By 40 years, 32% of patients had experienced a recurrence within the neck or at distant sites. Recurrence rates at 5, 10, 20, and 30 years were 20, 22, 27, and 30%, respectively. Alternatively expressed, the risk of recurrence (at any site) in this group of juvenile PTC patients treated with curative intent, approximated one in five at 5 years, one in four by 15 years, and one in three by 40 postoperative years. To date, in this cohort of 192 patients there have been 12 local (thyroid bed) recurrences, 38 regional (neck) nodal recurrences (discovered at least 180 days after initial surgery), and 13 distant metastases (discovered at least 30 days after surgery).
Postoperative neck nodal recurrences were found by 5, 10, and 20 years in 15, 17, and 21%, respectively. To date, 73% of first postoperative recurrences have been localized to regional neck nodes. At 5, 10, and 20 years the local recurrence rates were 3, 4, and 7%, respectively. Comparable rates for distant metastases were 4, 4, and 5%, respectively. Figure 3 illustrates the cumulative recurrence rates at each of these three sites. For the 171 patients who initially underwent bilateral lobar resection (BLR) with curative intent, the 20-year recurrence rates at local, regional, and distant sites were 3, 16, and 6%, respectively. Tumor recurrence (at any site) rates after BLR at 5, 10, and 20 years were 17, 19, and 22%, respectively.
Comparison of surgical outcome after UL or BLR
Twenty-four (96%) of the 25 patients who underwent UL had their surgery during the first three decades of the study period. During 1940–1969 UL accounted for 24% of the primary operations, while BLR occurred in 73%. Comparisons were made between the outcomes of the 24 UL patients and those of the 72 who underwent BLR during the same time period. Since there were only two deaths attributable to PTC, and both occurred in the larger group who underwent BLR, the statistically insignificant difference (P = 0.40) is probably not too meaningful. However, the numbers of events (11 local and 30 regional recurrences) and the group sizes (65 BLR and 20 UL with no gross residual) do permit comparisons in terms of local and regional recurrence rates. Figure 4 compares recurrence at local and regional sites between UL and BLR during 20 years. After BLR the local recurrence rate at 40 years was 6%. By contrast, the comparable rate after UL was significantly higher (P < 0.001) at 35%. Similarly, at 40 years the regional recurrence rates after BLR and UL were 13 and 60%, respectively (P < 0.0001). At 40 years tumor recurrence rates (at any site) after BLR and UL were 25 and 65%, respectively (P = 0.002).
During 1970–2008, 94% of the BLR procedures were either near-total (n = 59) or total (n = 51) thyroidectomies. Comparison was made between the two groups with respect to both cause-specific mortality and tumor recurrence rates. There were no deaths from PTC in either group during the period of study. No significant differences were found between the two procedures with respect to survival to recurrence at local (P = 0.42), regional (P = 0.10), or distant (P = 0.10) sites.
Impact of remnant ablation on outcome
Radioiodine remnant ablation (RRA) was not performed on any of the patients who had initial surgery during 1940 through 1949 since the FDA approved the use of I-131 as a radiopharmaceutical in 1951. However, during 1950 through 2008, 169 patients underwent BLR with curative intent, and RRA was administered within 6 months of initial surgery to 53 (31%) of this group. There was only one death in the nonablated group and none in those who underwent RRA (P = 0.62). Lower rates of recurrence at both local and distant sites were seen in those 53 who had been treated with both surgery and RRA, but the differences, compared to surgery alone (n = 116), were not significantly different (P = 0.18 and P = 0.13, respectively). With respect to the endpoint of neck nodal metastasis, the 20-year rate after surgery alone (16.5%) was not significantly different (P = 0.66) from that (15.5%) seen after surgery followed by RRA.
When similar data from the period of 1950–2008 were analyzed for the 161 patients who underwent either near-total or total thyroidectomy, there were no deaths due to PTC. The differences between survival after surgery alone and surgery + RRA (performed within 6 months of initial surgery) to endpoints of local recurrence (P = 0.18), regional (neck) nodal metastases (P = 0.61), locoregional recurrence (P = 0.37), distant metastases (P = 20), and recurrence at all sites (P = 0.27) were statistically insignificant.
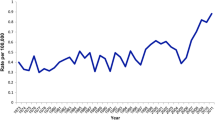
In pediatric practice it was often noted that there was some delay in administering postoperative RRA. We therefore decided to look at the efficacy of RRA given within 18 months of the initial surgery. During 1950–2008, RRA was administered within 18 months of initial surgery in 63 (32%) of the 198 cases. Figure 5 illustrates the varying frequency of its use during almost seven decades of RRA administration (within 18 months of surgery) in the cohort of 215 patients. When one considers the 169 patients treated by BLR with curative intent, 63 (37%) underwent RRA within 18 months from the date of initial definitive surgery. There was only one death in the surgery-alone group (P = 0.59). The differences between survival after surgery alone and surgery + RRA (performed within 18 months of initial surgery) to endpoints of local recurrence (P = 0.13), regional (neck) nodal metastases (P = 0.86), locoregional recurrence (P = 0.52), distant metastases (P = 0.06), and all-sites recurrence (P = 0.23) were shown to be statistically insignificant.
Lastly, comparison was made within the group treated by either TT or NT. There were no deaths due to PTC in either group. When the outcome after surgery alone (N = 100) and that of those also receiving RRA (N = 61) within 18 months of initial surgery were compared, the differences in survival to endpoints of local recurrence (P = 0.12), regional (neck) nodal recurrence (P = 0.79), locoregional recurrence (P = 0.46), and distant metastasis (P = 0.11) were statistically insignificant. Figure 6 compares survival through 40 years after initial surgery to the endpoint of regional (neck) nodal recurrence after either NT or TT alone (N = 100) or NT or TT + RRA (N = 61). With 26 total events and a cumulative 25-year regional (neck) nodal recurrence rate of 17%, this study had 80% power to detect an event rate hazard ratio (NT or TT alone vs. NT or TT + RRA) of 3.1, which corresponds to being able to detect a difference in cumulative event rates of 26 percentage points (17% vs. 43%).
Survival to all causes of death
During the period of study, 22 patients died. Figure 7 illustrates expected (E) and observed (O) survival to death (all causes). There appears to be no difference between E and O during the first 30 years, but between 30 and 50 years there appears to be an unexpected excessive death rate in this cohort of juvenile PTC patients. Using person-years estimates from a 1975 Minnesotan control population of identical age and sex, 11 deaths would have been expected. Using a one sample log-rank statistic test, this difference is very highly significant (P = 0.00045). Of the 22 patients who died, 17 (77%) died from malignancy. Of these 17 deaths, two were due to PTC and the remaining 15 (88%) died from other nonthyroid second primary malignancies (NSPM). Of the remaining five other fatalities, two were unrelated (drowning and murder) and three were attributed to cardiovascular disease (myocardial infarction).
Malignant causes of death in juvenile PTC
In the period of 1941–1950, there were 24 juvenile (range = 7–20 years, average = 14 years) PTC patients treated at Mayo. Fourteen survive and, to date, ten (42%) have died from malignancy: two directly from metastatic PTC and the other eight from NSPM. This period preceded the approval by the FDA of radioactive iodine I-131 as a radiopharmaceutical. Of the eight who died from NSPM, seven (87%) had received postoperative radium seed application or were given a course of X-ray therapy. Of these seven, four subsequently received I-131 in doses ranging from 175 to 200 mCi in addition to the prior irradiation. The sites of the radiation-associated NSPM were lung (3), trachea (1), pleura (1), and liver (1); the seventh had grade 4 adenocarcinoma of unknown origin (1). The eighth patient, who was 7 years old at diagnosis and received no adjunctive irradiation, died from cervical cancer at 40 years after PTC diagnosis.
During the radioiodine era of 1951–2008, 12 of the 191 patients have died, none of PTC. Of the seven who died from NSPM, five of the tumors arose from bone marrow (AML), duodenum, lung, breast, and brain (one each), while the other two had adenocarcinoma of uncertain origin. Four of these seven patients had received radioiodine. The AML patient had received 95 mCi and the lung cancer patient had received 150 mCi. One of the adenocarcinoma patients received 200 mCi, while the patient with breast cancer had received a cumulative dose of 1-131 of 840 mCi for her lung metastases found at PTC presentation. Of the 15 patients who died from NSPM, 11 (73%) had received postoperative therapeutic irradiation, which may prove relevant to NSPM pathogenesis.
Discussion
Papillary thyroid carcinoma and its follicular variant account for over 90% of all childhood thyroid cancers. It is a rare occurrence among children, accounting for less than 1.5% of all newly diagnosed childhood cancers and 7% of all head and neck tumors seen in children [23, 24]. The incidence is more than 10 times less (4 cases per million per year vs. 5 per 100,000) than in the adult population [25, 26].
Herein lies the challenge. Childhood PTC is a rare disease. Even at major centers worldwide, few such cancers are treated on an annual basis. It may take in excess of 30 years for a single institution to collect more than 100 cases from which to draw meaningful conclusions. During that time frame, practices and caregivers at the same institution change, making analysis of retrospective data a challenge, to say the least.
What do we know to date? Childhood PTC certainly has an excellent prognosis. Because of its rarity, indolent nature, and low death rates, randomized prospective therapeutic trials do not and likely never will exist [27].
To date, recommendations have been based on analysis of small cohorts, multicenter retrospective reviews, and extrapolations from adult studies and guidelines [2, 3, 5, 6, 25, 27–42]. In 2004, two of the authors (GBT, IDH), published a review article [27] on the subject, analyzing 21 worldwide studies that included nearly 1,800 children and adolescents. Management of such patients generally included a BLR, some form of gross nodal clearance based on palpable or visibly involved lymph nodes at the time of operation, routine RRA, and thyroid hormone suppressive therapy [1, 27, 43–47]. Despite this common thread, we at Mayo Clinic continue to embrace a more selective approach to the use of RAI in children, where ongoing concerns about potential risks and perceived benefits from its routine implementation continue to exist. It is for these reasons that we sought to review our single institutional experience with childhood PTC to better define outcomes: disease-related mortality, disease-free survival, operative morbidity, and death from second malignancies in children who received surgery alone versus surgery and radiation therapy.
It is stated that children often present with more advanced locoregional disease and distant metastases than their adult counterparts. The clinical presentation has, however, changed over the last several decades. In the past, nearly 50% of children had a history of ionizing radiation exposure to the head or neck. Today that figure, outside of the Chernobyl disaster region, has dropped to less than 3%. Palpable cervical adenopathy, once a common presentation, has decreased from 63 to 36%, invasion of contiguous structures from 31 to 6%, and distant (pulmonary) metastases from 19 to 6%. Presentation as a solitary thyroid nodule has increased from 37 to 73% among children. This may reflect increased awareness and surveillance but may also reflect a change in tumor biology in the post radiation-exposure era [25, 48–51].
Several authors have looked at specific factors that influence disease progression in children with PTC. Dinauer et al. [39] found multifocality, large tumor size, palpable nodes, and distant metastases to be significant but only in a univariate analysis; multifocality alone was significant in a multivariate analysis. Grigsby et al. [34] found that nodal disease was associated with disease progression in more than half and lung metastases in nearly one-third. T4 disease, residual gross tumor, distant metastases, and age under 15 were also associated with disease progression. Jarzab et al. [36] found in a multivariate analysis that less than TT was associated with disease progression. RAI had borderline impact. Landau et al. [37] found that patient age of less than 10 years and no thyroid hormone suppressive therapy (THST) were associated with increased risk of disease progression. In 1988, LaQuaglia et al. [5] found that age and histologic subtype were important determinants of disease progression. Twelve years later [38] they found that T4 and N1 disease, nodulectomy, type of node dissection, and incomplete tumor resection were most important. Newman et al. [40] described age and residual disease as important determinants of disease progression.
In large adult cohorts, lower tumor recurrence rates have been reported in patients treated with total thyroidectomy, I-131, and THST. Whether this leads to improved survival in children remains a matter of controversy [28, 38, 40, 51–62]. Brink et al. from Mayo Clinic [3] reviewed 14 children with PTC who were diagnosed with pulmonary metastases within 6 months of their original diagnosis. None have died after a median follow-up of 162 months. Zimmerman et al. [2] compared 58 children with 981 adults diagnosed and treated at Mayo Clinic from 1946 to 1975, with a median follow-up of more than 28 years. Cause-specific survival for both adults and children were identical for the first 30 postoperative years, but adults over age 40 at diagnosis had significantly higher mortality from PTC than did children (P < 0.0001). PTC in these children was more often metastatic to lymph nodes and lungs at presentation and more often recurrent in neck lymph nodes over time. Despite these differences in locoregional and distant metastases, PTC was less often fatal in children, possibly related to more DNA diploid tumors.
In this series of 215 patients younger than 21 years at diagnosis (median age = 16 years), the median duration of follow-up was nearly 30 years (5,838 patient-years of observation). Bilobar resection was performed in 87% of patients. Tumor excision was incomplete in 7%. Eighty-nine percent had no distant metastases within the first 30 days of the primary operation. Permanent hypoparathyroidism rates after TT were as high as 69, 56, and 25% in the 1950s, 1960s, and 1970s, respectively, but progressively decreased to 0% in those 26 patients treated by TT or NT in the new millennium (2000–2008). A better understanding of parathyroid preservation, routine autotransplantation of devitalized glands, meticulous attention to detail, magnifying loupes, and perhaps recurrent laryngeal nerve monitoring are likely causative factors in the witnessed diminished operative morbidity. Thyroid operations in children are technically challenging and should be performed by high-volume thyroid surgeons.
Management of regional nodal metastases in children has evolved over the 68-year time period from node-picking in the central and lateral neck to formal routine CCND and ultrasound-guided selective lateral neck dissection that began in the mid 1990s.
Postoperative RRA was performed in 35% of the 192 patients whose tumor at presentation was confined to the neck and who underwent complete tumor resection. In the entire cohort of patients (215), there were no deaths from PTC during the first 20 postoperative years and none of the 12 who presented with distant metastases died from PTC. Only two patients died of PTC: one 28 years and one 30 years following diagnosis. Both patients were diagnosed during 1948–1950 and had received external radiation at some point in their clinical course. There have been no deaths from PTC in the 181 patients treated since 1951 for an overall cause-specific survival of 98% at 50 years! Can we attribute this excellent survival to RRA? Not likely, given the fact that two-thirds of patients eligible for RAI did not receive radioiodine and yet did not succumb to PTC.
What about recurrence-free survival? By 40 years, 32% of patients had experienced a recurrence within the neck or at distant sites. Nodal recurrences accounted for two-thirds of all recurrences. What influenced recurrence-free survival: extent of surgery, RRA, or both? After BLR, the local recurrence rate at 40 years was 6%, significantly better than that seen for unilateral lobectomy (35%, P < 0.001). Did the addition of RRA have any impact on lowering recurrence rates? Although rates of recurrence at both local and distant sites were somewhat lower in the 53 patients receiving surgery and RRA compared to that those after surgery alone (116 patients), there was no statistically significant difference. When looking specifically at neck nodal metastases, the 20-year rate of recurrence with surgery alone was 16.5 and 15.5% when RRA was added (P = 0.66).
In summary, the differences between survival after surgery alone and after surgery plus RRA to the endpoints of local recurrence, neck nodal metastases, locoregional recurrence, distant metastases, and all-sites recurrence were shown in this large, single institutional cohort to be statistically insignificant.
What are the arguments then for routine RRA? True, total thyroidectomy can be difficult to achieve. Meaningful remnant uptake on whole-body scanning can be significant, even after a so-called “total thyroidectomy.” Metastases (locoregional and distant, more common in children) could theoretically be missed without prior RRA. RRA does facilitate whole-body radioiodine scanning, the subsequent delivery of therapeutic radioiodine doses, and possibly allows an easier interpretation of stimulated serum thyroglobulin (Tg) levels as a tumor marker. However, the data presented in our study suggests that we can very successfully manage PTC in childhood and adolescence without RRA. Since 1951, when I-131 was introduced to our practice, we have not seen a death from childhood PTC and all-sites recurrences have not been impacted by the addition of postoperative RRA to initial definitive surgery. An upward trend in an autoantibody-negative serum Tg level on THST, measured in today’s sensitive assays (with detection limits of 0.1 ng/ml or less), is more than sufficient justification to step up the search for regional or distant metastatic disease in these patients with very well differentiated and Tg-producing tumors utilizing high-resolution neck ultrasound (the best tool for identifying recurrent/residual disease in the neck,) along with the selective use of thin-cut (1.25-mm slices), non-contrast CT examination of the chest.
An unexpected finding in this study was the significant number of premature deaths in this cohort from a second primary malignancy. Using a Minnesotan control population, 11 deaths would have been expected during this time period. Twice as many deaths were encountered (n = 22), prematurely killing patients between 30 and 50 years after their original diagnosis of PTC. Seventeen patients (77%) died of malignancy, two from PTC and the remainder of NSPM. The occurrence of second non-PTC malignancies in this cohort is statistically significant (P = 0.00045) and most concerning, to say the least. Of the 15 patients who died from NSPM, 11 (73%) had received postoperative therapeutic irradiation, including external radiation, radium seed application, radioiodine, or a combination thereof. Although one cannot implicate radioiodine as clearly causative, in and of itself, the potential implication would be that all forms of ionizing radiation may be carcinogenic to childhood thyroid cells. In the absence of a beneficial effect, except for patients with incomplete resection and distant metastases, all forms of therapeutic irradiation in this setting would hardly seem beneficial and probably not warranted.
What do we know about the effects of radioiodine? Early side effects of I-131 include painful swelling of remnant tissue or metastases. Nausea, vomiting, sialadenitis, and transient loss of taste or smell all may occur. Sialadenitis can be permanent, resulting in significant dental deterioration. Transient bone marrow depression with leucopenia and thrombocytopenia can occur. Azoospermia has been reported in adults receiving total cumulative doses of greater than 300 mCi [63]. I-131 has been associated with an increased risk of miscarriage during the first year post-treatment, but the question of whether testicular irradiation from I-131 is linked to impaired fertility or consequences to offspring remains to be established [64]. Total cumulative doses of over 500 mCi in children and 800 mCi in adolescents appear associated with a higher lifetime risk of leukemia [65].
One could argue that the appearance of NSPM in this cohort may not reflect the mutagenic effects of radiation but may denote a predisposition to tumor formation in a group of patients who have already developed a rare cancer at a young age. We cannot refute that, except to say that the tumors seen were encountered more frequently in the group exposed to ionizing radiation (2/3), compared to only one-third of those in the nonirradiated group. Certainly both mechanisms (overzealous treatment versus tumor predisposition) may play a role [14, 66].
Conclusions
Pediatric PTC is a rare disorder. Cause-specific mortality is likely less than 1% in the modern era. High-volume surgeons can carry out a safe TT or NTT, routine CCND, and an ultrasound-guided selective lateral neck dissection with minimal morbidity in most cases. This initial surgical approach has the greatest impact on all-sites recurrence and is not further influenced by the addition of RRA. Of greater concern is the occurrence of NSPM deaths 30–50 years after initial diagnosis, primarily in patients subjected to some form of adjuvant ionizing radiation. We strongly recommend that radioiodine therapy in childhood PTC be utilized only selectively in high-risk patients such as those with distant metastases or after incomplete surgical resection of primary tumor, with gross residual disease. In this regard, we are in agreement with our European colleagues [66] who found in adults an increased risk of NSPM with increasing cumulative activity of I-131 administered and concluded that “these results strongly highlight the necessity to delineate the indication for I-131 treatment in thyroid cancer patients in order to restrict its use to patients in whom clinical benefits are expected.” These conclusions would seem particularly pertinent to a population younger than 21 years of age at first therapy, who would be predicted to live for at least 50 years after diagnosis.
References
Hogan AR, Zhuge Y, Perez EA et al (2009) Pediatric thyroid carcinoma: Incidence and outcomes in 1753 patients. J Surg Res 156(1):167–172
Zimmerman D, Hay ID, Gough IR et al (1988) Papillary thyroid carcinoma in children and adults: long-term follow-up of 1039 patients treated at one institution during three decades. Surgery 104:1157–1166
Brink J, van Heerden J, McIver B et al (2000) Papillary thyroid cancer with pulmonary metastases in children: long-term prognosis. Surgery 128:881–886
Crile G (1959) Carcinoma of the thyroid in children. Ann Surg 150:959–964
LaQuaglia MP, Corbally MT, Heller G et al (1988) Recurrence and morbidity in differentiated thyroid carcinoma in children. Surgery 104:1149–1156
Ceccarelli C, Pacini F, Lippi F et al (1988) Thyroid cancer in children and adolescents. Surgery 104:1143–1148
Demidchik YE, Demidchik EP, Reiners C et al (2006) Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg 243:525–532
Hallwirth U, Flores J, Kaserer K et al (1999) Differentiated thyroid cancer in children and adolescents: the importance of adequate surgery and review of literature. Eur J Pediatr Surg 9:359–363
Handkiewicz-Junak D, Wloch J, Roskosz J et al (2007) Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med 48:879–888
Rachmiel M, Charron M, Gupta A et al (2006) Evidence-based review of treatment and followup of pediatric patients with differentiated thyroid carcinoma. J Pediatr Endocrinol Metab 19:1377–1393
Hay ID, Thompson GB, Grant CS et al (2002) Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 26:879–885
Hay ID, McDougall IR, Sisson JC (2008) Perspective: the case against radioiodine remnant ablation in patients with well-differentiated thyroid carcinoma. J Nucl Med 49:1395–1397
Hay ID, McConahey WM, Goellner JR (2002) Managing patients with papillary thyroid carcinoma. Insights gained from the Mayo Clinic’s experience of treating 2, 521 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc 113:241–260
Vassilopoulou-Sellin R, Goepfert H, Raney B et al (1998) Differentiated thyroid cancer in children and adolescents: clinical outcome and mortality after long-term follow-up. Head Neck 20:549–555
LiVolsi VA, Albores-Saavedra J, Asa SL (2004) Papillary carcinoma. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C et al (eds) Pathology and genetics of tumours of endocrine organs: WHO Classification of Tumours. IARC Press, Lyon, pp 57–66
McConahey WM, Hay ID, Woolner LB et al (1986) Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy and outcome. Mayo Clin Proc 61:978–996
Peto R, Peto J (1972) Asymptotically efficient rank invariant procedures (with discussion). J R Stat Soc Ser A 135:185–207
SAS Institute (1990) SAS/STAT user’s guide, version, 6th edn. SAS Institute, Cary
Hay ID, Bergstralh EJ, Goellner JR et al (1993) Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1, 779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1058
Cady B (1997) Our AMES is true: how an old concept still hits the mark: or, risk group assignment points the arrow to rational therapy selection in differentiated thyroid cancer. Am J Surg 174:462–468
Hay ID, Grant CS, Taylor WF et al (1987) Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic system. Surgery 102:1088–1095
Hay ID, Hutchinson ME, Gonzalez-Losada T et al (2008) Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery 144:980–988
Hung W, Sarlis NJ (2002) Current controversies in the management of pediatric patients with well-differentiated nonmedullary thyroid cancer: a review. Thyroid 12:683–702
Bernstein L, Gurney JG (1999) Carcinomas and other malignant epithelial neoplasms. In: Ries LAG, Smith MA, Gurney JG et al (eds) Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. National Cancer Institute, NIH Pub No 99-4649, Bethesda, Maryland, pp 139–147
Harness JK (1997) Childhood thyroid carcinoma. In: Clark OH, Duh Q-Y (eds) Textbook of endocrine surgery. Saunders, Philadelphia, pp 75–81
Young JL, Percy CL, Asire AJ et al (1981) Cancer incidence and mortality in the United States, 1973–1977. Natl Cancer Inst Monogr 57:1
Thompson GB, Hay ID (2004) Current strategies for surgical management and adjuvant treatment of childhood papillary thyroid carcinoma. World J Surg 28:1187–1198
Schlumberger M, De Vathaire F, Travagli JP et al (1987) Differentiated thyroid carcinoma in childhood: long-term follow-up of 72 patients. J Clin Endocrinol Metab 65:1088–1094
Jacob P, Goulko G, Heidenreich WF et al (1998) Thyroid cancer risk to children calculated. Nature 392:31–32
Haveman JW, van Tol KM, Rouwe CW et al (2003) Surgical experience in children with differentiated thyroid carcinoma. Ann Surg Oncol 10:15–20
Kowalski LP, Filho JG, Pinto CAL et al (2003) Long-term survival rates in young patients with thyroid carcinoma. Arch Otolaryngol Head Neck Surg 129:746–749
Giuffrida D, Scollo C, Pellegriti G et al (2002) Differentiated thyroid carcinoma in children and adolescents. J Endocrinol Invest 25:18–24
Arici C, Erdogan O, Altunbas H et al (2002) Differentiated thyroid cancer in children and adolescents: clinical characteristics, treatment, and outcome of 15 patients. Horm Res 57:153–156
Grigsby PW, Gal-or A, Michalski JM et al (2002) Childhood and adolescent thyroid carcinoma. Cancer 95:724–729
Ben Arush MW, Stein ME, Nahum MP et al (2000) Pediatric thyroid carcinoma: 22 years of experience at the Northern Israel Oncology Center (1973–1995). Pediatr Hematol Oncol 17:75–92
Jarzab B, Junak DH, Wloch J et al (2000) Multivariate analysis of prognostic factors for differentiated thyroid carcinoma in children. Eur J Nucl Med 35:955–960
Landau D, Vini L, Ahern R et al (2000) Thyroid cancer in children: the Royal Marsden Hospital experience. Eur J Cancer 36:214–220
LaQuaglia MP, Black T, Holcomb GW et al (2000) Differentiated thyroid cancer: clinical characteristics, treatment, and outcome in patients under 21 years of age who present with distant metastases: a report from the Surgical Discipline Committee of the Children’s Cancer Group. J Pediatr Surg 35:955–960
Dinauer CA, Tuttle RM, Robie DK et al (1997) Clinical features associated with metastasis and recurrence of differentiated thyroid cancer in children, adolscents, and young adults. Clin Endocrinol (Oxf) 49:619–628
Newman KD, Black T, Heller G et al (1998) Differentiated thyroid cancer: determinants of disease progression in patients <21 years of age at diagnosis: a report from the Surgical Discipline Committee of the Children’s Cancer Group. Ann Surg 227:533–541
Segald K, Shvero J, Stern Y et al (1998) Surgery of thyroid cancer in children and adolescents. Head Neck 20:293–297
Vassilopoulou-Sellin R, Schultz PN, Haynie TP (1996) Clinical outcome of patients with papillary thyroid carcinoma who have recurrence after initial radioactive iodine therapy. Cancer 78:493–501
Josefson J, Zimmerman D (2008) Thyroid nodules and cancers in children. Pediatr Endocrinol Rev 6(1):14–23
Dinauer CA, Breuer C, Rivkees SA (2008) Differentiated thyroid cancer in children: diagnosis and management. Curr Opin Oncol 20:59–65
Gingalewski CA, Newman KD (2006) Seminars: controversies in the management of pediatric thyroid malignancy. J Surg Oncol 94:748–752
Collini P, Mattavelli F, Spinelli C et al (2007) Treatment of sporadic nonmedullary thyroid carcinomas in pediatric age. Expert Rev Anticancer Ther 7:23–30
Collini P, Massimino M, Leite SF et al (2006) Papillary thyroid carcinoma of childhood and adolescence: a 30-year experience at the Istituto Nazionale Tumori in Milan. Pediatr Blood Cancer 46:300–306
Kirkland RT, Kirkland JL, Rosenberg HS (1973) Solitary thyroid nodules in 30 children and report of a child with a thyroid abscess. Pediatrics 51:85
Harness JK, Thompson NW, Nishiyama RH (1971) Childhood thyroid carcinoma. Arch Surg 102:278
Fowler CL, Pokorny WJ, Harberg FJ (1989) Thyroid nodules in children: current profile of changing disease. South Med J 82:1472
Harness JK, Thompson NW, McLeod MK et al (1992) Differentiated thyroid carcinoma in children and adolescents. World J Surg 16:547–554
Mazzaferri EL (1999) NCCN thyroid carcinoma practice guidelines. Oncology 13:391–416
Paryani SB, Chobe RJ, Scott W et al (1996) Management of thyroid carcinoma with radioactive I-131. Int J Radiat Oncol Biol Phys 36:S83–S86
Gorlin JB, Sallen SE (1990) Thyroid cancer in childhood. Endocrinol Metab Clin North Am 19:649–662
Desjardins JG, Bass J, Leboeuf G et al (1988) A twenty-year experience with thyroid carcinoma in children. J Pediatr Surg 23:709–713
McClellan DR, Francis GL (1996) Thyroid cancer in children, pregnant women, and patients with Graves’ disease. Endocrinol Metab Clin North Am 25:27–48
Welch Dinauer CA, Tuttle RM, Robie DK et al (1998) Clinical features associated with metastasis and recurrence of differentiated thyroid cancer in children, adolescents, and young adults. Clin Endocrinol (Oxf) 49:619–628
Sykes AJ, Gattamanen HR (1997) Carcinoma of the thyroid in children: a 25-year experience. Med Pediatr Oncol 29:103–107
Fassina AS, Rupolo M, Pelizzo MR et al (1994) Thyroid cancer in children and adolescents. Tumori 80:257–262
Danese D, Gardini A, Farsetti A et al (1997) Thyroid carcinoma in children and adolescents. Eur J Pediatr 156:190–194
Dottorini ME, Vignati A, Mazzucchelli L et al (1997) Differentiated thyroid carcinoma in children and adolescents: a 37-year experience in 85 patients. J Nucl Med 38:669–675
Yeh SD, La Quaglia MP (1997) I-131 therapy for pediatric thyroid cancer. Semin Pediatr Surg 6:128–133
Winters S, Berga S (1997) Gonadal dysfunction in patients with thyroid disorders. Endocrinologist 7:167–173
Garsi JP, Schlumberger M, Ricard M et al. (2009) Health outcomes of children fathered by patients treated with radioiodine for thyroid cancer. Clin Endocrinol (Oxf) (Epub ahead of print)
Sisson JC (1989) Medical treatment of benign and malignant thyroid tumors. Endocrinol Metab Clin North Am 18:359–387
Rubino C, de Vathaire F, Dottorini ME et al (2003) Second primary malignancies in thyroid cancer patients. Br J Cancer 89:1638–1644
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hay, I.D., Gonzalez-Losada, T., Reinalda, M.S. et al. Long-Term Outcome in 215 Children and Adolescents with Papillary Thyroid Cancer Treated During 1940 Through 2008. World J Surg 34, 1192–1202 (2010). https://doi.org/10.1007/s00268-009-0364-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-009-0364-0