Abstract
Background
Experimental and clinical studies have demonstrated the pivotal role of oxidative stress in the promotion of hepatic and intestinal injury in obstructive jaundice. The present study was undertaken to investigate the effect of well known antioxidant treatments on the gut–liver axis oxidative status and function in bile duct-ligated rats.
Methods
A total of 60 male Wistar rats were randomly divided into six groups of 10 animals each: controls, sham operated, bile duct ligated (BDL), and BDL treated with either N-acetylcysteine (NAC), allopurinol, or α-tocopherol (α-TC). Ten days after treatment, the hepatic and intestinal oxidative status was estimated by measuring lipid peroxidation and a battery of biochemical markers comprising the organ’s thiol redox state (i.e., glutathione, cysteine, protein thiols, oxidized glutathione, nonprotein mixed disulfides, oxidized cysteine derivatives, protein symmetrical disulfides, and protein mixed disulfides). Portal and aortic endotoxin concentrations and alanine aminotransferase (ALT) levels were also determined.
Results
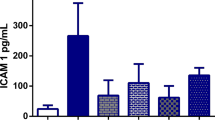
All antioxidant treatments significantly improved intestinal barrier function and protected from cholestatic liver injury, as evidenced by reduction of the portal and aortic endotoxin concentration and ALT levels, respectively. This effect accompanied their significant antioxidant action in both organs, mediated by a certain influence profile on the thiol redox state by each treatment.
Conclusion
NAC, allopurinol, and α-TC, exerting a potent combined antioxidant effect on the intestine and liver in experimental obstructive jaundice, significantly prevented intestinal barrier dysfunction and liver injury. The variety of results depending on the antioxidant agent that was administered and the marker of oxidative stress that was estimated, indicates that a battery of biomarkers would be more appropriate in assessing pharmacologic responses to therapeutic interventions.


Similar content being viewed by others
References
Pain JA, Cahill CJ, Bailey ME (1985) Perioperative complications in obstructive jaundice: therapeutic considerations. Br J Surg 72(12):942–945
Clements WD, Parks R, Erwin P, et al. (1996) Role of the gut in the pathophysiology of extrahepatic biliary obstruction. Gut 39(4):587–593
Krahenbuhl S, Talos C, Fischer S, et al. (1994) Toxicity of bile acids on the electron transport chain of isolated rat liver mitochondria. Hepatology 19(2):471–479
Brown KM, Brems JJ, Moazzam FN, et al. (2003) The nitric oxide donor molsidomine improves survival and reduces hepatocyte apoptosis in cholestasis and endotoxemia. J Am Coll Surg 197(2):261–269
Liu TZ, Lee KT, Chern CL, et al. (2001) Free radical-triggered hepatic injury of experimental obstructive jaundice of rats involves overproduction of proinflammatory cytokines and enhanced activation of nuclear factor kappaB. Ann Clin Lab Sci 31(4):383–390
Assimakopoulos SF, Vagianos CE, Zervoudakis G, et al. (2004) Gut regulatory peptides bombesin and neurotensin reduce hepatic oxidative stress and histological alterations in bile duct ligated rats. Regul Pept 120(1–3):185–193
Vendemiale G, Grattagliano I, Lupo L, et al. (2002) Hepatic oxidative alterations in patients with extra-hepatic cholestasis: effect of surgical drainage. J Hepatol 37(5):601–605
Assimakopoulos SF, Vagianos CE, Patsoukis N, et al. (2004) Evidence for intestinal oxidative stress in obstructive jaundice-induced gut barrier dysfunction in rats. Acta Physiol Scand 180(2):177–185
Assimakopoulos SF, Thomopoulos KC, Patsoukis N, et al. (2006) Evidence for intestinal oxidative stress in patients with obstructive jaundice. Eur J Clin Invest 36(3):181–187
Ljubuncic P, Tanne Z, Bomzon A (2000) Evidence of a systemic phenomenon for oxidative stress in cholestatic liver disease. Gut 47(5):710–716
Patsoukis N, Georgiou CD (2004) Determination of the thiol redox state of organisms: new oxidative stress indicators. Anal Bioanal Chem 378(7):1783–1792
Ocal K, Avlan D, Cinel I, et al. (2004) The effect of N-acetylcysteine on oxidative stress in intestine and bacterial translocation after thermal injury. Burns 30(8):778–784
Miyazono Y, Gao F, Horie T (2004) Oxidative stress contributes to methotrexate-induced small intestinal toxicity in rats. Scand J Gastroenterol 39(11):1119–1127
Nakano H, Boudjema K, Jaeck D, et al. (1996) Amelioration of hepatocellular integrity and inhibition of sinusoidal oxidative stress by N-acetylcysteine pretreatment in cold ischemia-reperfusion injury of rat liver. Eur Surg Res 28(4):245–255
Kang SM, Lim S, Song H, et al. (2006) Allopurinol modulates reactive oxygen species generation and Ca2+ overload in ischemia-reperfused heart and hypoxia-reoxygenated cardiomyocytes. Eur J Pharmacol 535(1–3):212–219
Czako L, Takacs T, Varga IS, et al. (2000) Oxidative stress in distant organs and the effects of allopurinol during experimental acute pancreatitis. Int J Pancreatol 27(3):209–216
Grisham MB, Hernandez LA, Granger DN (1986) Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol 251(4 Pt 1):G567–G574
Terzi C, Kuzu A, Aslar AK, et al. (2001) Prevention of deleterious effects of reperfusion injury using one-week high-dose allopurinol. Dig Dis Sci 46(2):430–437
Rhoden E, Pereira-Lima L, Lucas M, et al. (2000) The effects of allopurinol in hepatic ischemia and reperfusion: experimental study in rats. Eur Surg Res 32(4):215–222
Machlin LJ, Bendich A (1987) Free radical tissue damage: protective role of antioxidant nutrients. FASEB J 1(6):441–445
Packer L, Landvik S (1989) Vitamin E: introduction to biochemistry and health benefits. Ann N Y Acad Sci 570:1–576
Mutlu-Turkoglu U, Erbil Y, Oztezcan S, et al. (2000) The effect of selenium and/or vitamin E treatments on radiation-induced intestinal injury in rats. Life Sci 66(20):1905–1913
Horwitt MK (1991) Data supporting supplementation of humans with vitamin E. J Nutr 121(3):424–429
Rocksen D, Ekstrand-Hammarstrom B, Johansson L, et al. (2003) Vitamin E reduces transendothelial migration of neutrophils and prevents lung injury in endotoxin-induced airway inflammation. Am J Respir Cell Mol Biol 28(2):199–207
Nakano H, Fujiwara Y, Kitamura N, et al. (2000) Susceptibility to lipopolysaccharide of cholestatic rat liver produced with bile duct ligation: assessments of the mitochondrial glutathione pool and the effects of N-acetylcysteine. Eur Surg Res 32(3):148–154
Mun KC, Kwak CS, Kwon KY (1996) The protective effect of allopurinol on cholestatic liver injury induced by bile duct ligation. J Korean Med Sci 11(3):239–243
Schimpl G, Pesendorfer P, Steinwender G, et al. (1996) Allopurinol reduces bacterial translocation, intestinal mucosal lipid peroxidation, and neutrophil-derived myeloperoxidase activity in chronic portal hypertensive and common bile duct-ligated growing rats. Pediatr Res 40(3):422–428
Schimpl G, Pesendorfer P, Steinwender G, et al. (1997) The effect of vitamin C and vitamin E supplementation on bacterial translocation in chronic portal hypertensive and common-bile-duct-ligated rats. Eur Surg Res 29(3):187–194
Husain K, Mejia J, Lalla J, Kazim S (2005) Dose response of alcohol-induced changes in BP, nitric oxide and antioxidants in rat plasma. Pharmacol Res 51(4):337–343
Yilmaz S, Yilmaz E (2006) Effects of melatonin and vitamin E on oxidative-antioxidative status in rats exposed to irradiation. Toxicology 222(1–2):1–7
Assimakopoulos SF, Scopa CD, Zervoudakis G, et al. (2005) Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann Surg 241(1):159–167
Assimakopoulos SF, Scopa CD, Charonis A, et al. (2004) Experimental obstructive jaundice disrupts intestinal mucosal barrier by altering occludin expression: beneficial effect of bombesin and neurotensin. J Am Coll Surg 198(5):748–757
Neuschwander-Tetri BA, Nicholson C, Wells LD, et al. (1996) Cholestatic liver injury down-regulates hepatic glutathione synthesis. J Surg Res 63(2):447–451
Singh S, Shackleton G, Ah-Sing E, et al. (1992) Antioxidant defenses in the bile duct-ligated rat. Gastroenterology 103(5):1625–1629
Sakaguchi S, Furusawa S, Yokota K, et al. (1996) The enhancing effect of tumour necrosis factor-alpha on oxidative stress in endotoxemia. Pharmacol Toxicol 79(5):259–265
Pata C, Caglikulekci M, Cinel L, et al. (2002) The effects of antithrombin-III on inducible nitric oxide synthesis in experimental obstructive jaundice: an immunohistochemical study. Pharmacol Res 46(4):325–331
Unno N, Wang H, Menconi MJ, et al. (1997) Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology 113(4):1246–1257
Tsuji K, Kubota Y, Yamamoto S, et al. (1999) Increased neutrophil chemotaxis in obstructive jaundice: an in vitro experiment in rats. J Gastroenterol Hepatol 14(5):457–463
Tsai LY, Lee KT, Lu FJ (1997) Biochemical events associated with ligation of the common bile duct in Wistar rats. J Formos Med Assoc 96(1):17–22
Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30(11):1191–1212
Dogterom P, Mulder GJ, Nagelkerke JF (1989) Lipid peroxidation-dependent and -independent protein thiol modifications in isolated rat hepatocytes: differential effects of vitamin E and disulfiram. Chem Biol Interact 71(2–3):291–306
Wu Y, Wang F, Zheng Q, et al. (2006) Hepatoprotective effect of total flavonoids from Laggera alata against carbon tetrachloride-induced injury in primary cultured neonatal rat hepatocytes and in rats with hepatic damage. J Biomed Sci 13(4):569–578
Zhang Z, Leonard SS, Huang C, et al. (2003) Role of reactive oxygen species and MAPKs in vanadate-induced G2/M phase arrest. Free Radic Biol Med 34(10):1333–1342
Kamata H, Hirata H (1999) Redox regulation of cellular signalling. Cell Signal 11(1):1–14
Yang R, Harada T, Li J, et al. (2005) Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase 1/2 dependent mechanism. Intensive Care Med 31(5):709–717
Rao RK, Basuroy S, Rao VU, et al. (2002) Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368(Pt 2):471–481
Deitch EA, Ma L, Ma WJ, et al. (1989) Inhibition of endotoxin-induced bacterial translocation in mice. J Clin Invest 84(1):36–42
Deitch EA (1992) Multiple organ failure: pathophysiology and potential future therapy. Ann Surg 216(2):117–134
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Assimakopoulos, S.F., Maroulis, I., Patsoukis, N. et al. Effect of Antioxidant Treatments on the Gut–Liver Axis Oxidative Status and Function in Bile Duct-Ligated Rats. World J Surg 31, 2023–2032 (2007). https://doi.org/10.1007/s00268-007-9191-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-007-9191-3