Abstract
Background
We examined the relationship between different expressions of positive axillary lymph nodes (PN) and the outcomes of node-positive breast carcinoma patients to determine the best predictor(s) among these expressions and to assess whether anatomic high level involvement is an independent prognostic factor.
Study Design
In this retrospective study, the primary endpoints were distant recurrence (DR), locoregional recurrences (LRR), and disease-free survival (DFS). Univariate and multivariate prognostic factor analyses were carried out using survival and regression methods in the data of 704 patients with PN.
Results
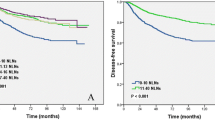
In multivariate analysis, the number of PN, ratio of PN, log odds of PN, and level III (L-III) involvement, separately, were significant factors for DR in addition to age, tumor size, and lymphovascular invasion (LVI). In the final model including all expressions of nodal involvement, age (continuous P = 0.001; hazard ratio [HR]: 0.98; 95% confidence Interval [95% CI]: 0.96–0.99), tumor size (continuous: P < 0.0001; HR: 1.3; 95% CI, 1.2–1.5), LVI (yes vs. no: P = 0.005; HR: 1.6; 95% CI, 1.2–2.2), and ratio of PN (continuous: P = 0.02; HR: 1.03; 95% CI, 1.01–1.06) were the independent prognostic factors for DR. For LRR, ratio of PN (continuous: P = 0.001; HR: 1.02; 95% CI, 1.01–1.03) was the most important factor in addition to age (continuous: P = 0.02; HR: 0.98; 95% CI, 0.97–0.99) and tumor size (continuous: P = 0.04; HR: 1.3; 95% CI, 1.1–1.6). When patients were stratified by number categories of PN (1–3 vs. 4–9 vs. ≥ 10), there was no difference between DFSs of patients with and without L-III involvement. In contrast, when patients were stratified by L-III involvement, DFSs according to the number categories were statistically different.
Conclusions
Ratio of PN was more valuable than number of PN for predicting outcome in node-positive breast carcinoma patients. Level III involvement was not an independent prognostic indicator either for locoregional or for distant recurrences.



Similar content being viewed by others
References
Hadjiloucas I, Bundred NJ. Axillary surgery: is it necessary? Breast 2000;9:2–4
Bembenek A, Schlag PM. Lymph node dissection in breast cancer. Langenbecks Arch Surg 2000;385:236–245
Canavese G, Catturich A, Vecchio C, et al. Prognostic role of lymph node level involvement in patients undergoing axillary dissection for breast cancer. Eur J Surg Oncol 1998;24:104–109
Vin-Hung V, Verschraegen C, Promish DI, et al. Ratios of involved nodes in breast cancer. Breast Cancer Res 2004;6:R680–R688
Toma S, Leonessa F, Romanini A, et al. Predictive value of some clinical and pathological parameters on upper level axillary lymph node involvement in breast cancer. Anticancer Res 1991;11:1439–1444
Newman LA, Kuerer HM, Fornage B, et al. Adverse prognostic significance of infraclavicular lymph nodes detected by ultrasonography in patients with locally advanced breast cancer. Am J Surg 2001;181:313–318
Barth RJ, Danforth DN, Venzon DJ, et al. Level of axillary involvement by lymph node metastases from breast cancer is not an independent predictor of survival. Arch Surg 1991;126:574–577
Haffty BG, Ward B, Pathare P, et al. Reappraisal of the role of axillary lymph node dissection in the conservative treatment of breast cancer. J Clin Oncol 1997;15:691–700
Luini A, Zurrida S, Galimberti V, et al. Axillary dissection in breast cancer. Crit Rev Oncol Hematol 1999;30:63–70
Sinha PS, Thrush S, Bendall S,et al. Does radical surgery to the axilla give a survival advantage in more severe breast cancer? Eur J Cancer 2002;38:1474–1477
Voss M, Schneider JW, Apffelstaedt J. Axillary lymph node involvement in stage III breast cancer: treatment implications. J Surg Oncol 1999;71:162–166
Greene FL, Page DL, Fleming ID, et al. editors. AJCC Cancer Staging Manuel. 6th ed. Berlin Heidelberg New York: Springer, 2002
Megale Costa LJ, Soares HP, Gaspar HA, et al. Ratio between positive lymph nodes and total dissected axillaries lymph nodes as an independent prognostic factor for disease-free survival in patients with breast cancer. Am J Clin Oncol 2004;27:304–306
Truong PT, Berthelet E, Lee J, et al. The prognostic significance of the percentage of positive/dissected axillary lymph nodes in breast cancer recurrence and survival in patients with one to three positive axillary lymph nodes. Cancer 2005;103:2006–2014
Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc 1958;53:457–481
Cox DR. Regression models and life tables. J R Stat Soc 1972;B34:187–220
Kleinbaum DG, Kupper LL, Muller KE, et al. editors. Applied Regression Analysis and Other Multivariable Methods, 3rd edn. Pacific Grove: Brooks/Cole, 1998
Harrell FE Jr. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. Berlin Heidelberg New York: Springer, 2001
Schemper M. Predictive accuracy and explained variation. Stat Med 2003;22:2299–2308
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver characteristic (ROC) curve. Radiology 1982;143:29–36
Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35
Moore MP, Kinne DW. Axillary lymphadenectomy: a diagnostic and therapeutic procedure. J Surg Oncol 1997;66:2–6
Jansen RLH, Hillen HFP, Schouten HC. Prognostic and predictive factors in breast cancer. Netherlands J Med 1997;51:65–77
Joslyn SA, Konety BR. Effect of axillary lymphadenectomy on breast carcinoma survival. Breast Cancer Res Treat 2005;91:11–18
Sakorafas GH, Tsiotou AG, Balsiger BM. Axillary lymph node dissection in breast cancer. Acta Oncol 2000;39:455–466
Saha S, Farrar WB, Young DC, et al. Variation in axillary node dissection influences the degree of nodal involvement in breast cancer patients. J Surg Oncol 2000;73:134–137
Recht A. Nodal treatment for patients with early stage breast cancer: guilty or innocent? Radiother Oncol 1992;25:79–82
Yildirim E, Soydinc P, Yildirim N, et al. Role of increased arterial inflow in arm edema after modified radical mastectomy. J Exp Clin Cancer Res 2000;19:427–430
Chua B, Ung O, Taylor R, et al. Is there a role for axillary dissection for patients with operable breast cancer in this era of conservatism? ANZ J Surg 2002;72:786–792
Petrek JA, Heelan MC. Incidence of breast carcinoma related lymphedema. Cancer 1998;83:2776–2781
Liljegren G, Holmberg L. Arm morbidity after sector resection, axillary dissection with or without postoperative radiotherapy in breast cancer stage I: results from a randomized trial—Uppsala–Orebro Breast Cancer Study Group. Eur J Cancer 1997;33:193–199
Kuehn T, Klas W, Darsow M, et al. Long-term morbidity following axillary dissection in breast cancer patients: clinical assessment, significance for life quality and the impact of demographic, oncologic, and therapeutic factors. Breast Cancer Res Treat 2000;64:275–286
Taylor KO. Morbidity associated with axillary surgery for breast cancer. ANZ J Surg 2004;74:314–317
Somner JEA, Dixon JMJ, Thomas JSJ. Node retrieval in axillary lymph node dissections: recommendations for minimum numbers to be confident about node negative status. J Clin Pathol 2004;57:845–848
Blichert-Toft M. Axillary surgery in breast cancer management. Acta Oncol 2000;39:269–275
Axelsson CK, Mouridsen HT, Zedeler K. Axillary dissection of level I and II lymph nodes is important in breast cancer classification. The Danish Breast Cancer Cooperative Group (DBCG).. Eur J Cancer 1992;28A:1415–1418
Petrik DW, McCready DR, Sawka CA, Goel V. Association between extent of axillary lymph node dissection and patient, tumor, surgeon, and hospital factors in patients with early breast cancer. J Surg Oncol 2003;82:84–90
Cady B. A contemporary view of axillary dissection. Ann Surg 2000;232:8–9
Rutgers EJ, EUSOMA Consensus Group. Quality control in the locoregional treatment of breast cancer. Eur J Cancer 2001;37:447–453
Rutgers EJ. Guidelines to assure quality in breast cancer surgery. Eur J Surg Oncol 2005;31:568–576
Sosa JA, Diener-West M, Gusev Y, et al. Association between extent of axillary lymph node dissection and survival in patients with stage I breast cancer. Ann Surg Oncol 1998;5:140–149
Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: International consensus panel on the treatment of primary breast cancer. J Clin Oncol 2001;19:3817–3827
Goldhirsch A, Glick JH, Gelber RD, et al, and panel members. Meeting highlights: International expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 2005;16:1569–1583
Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1539–1569
Kingsmore DB, Ssemwogerere A, Hole DJ, et al. Increased mortality from breast cancer and inadequate axillary treatment. Breast 2003;12:36–41
Gervasoni JE, Taneja C, Chung MA, et al. Axillary dissection in the context of the biology of lymph node metastases. Am J Surg 2000;180:278–283
Pierce LJ. The use of radiotherapy after mastectomy: A review of the literature. J Clin Oncol 2005;23:1706–1717
Recht A, Gray R, Davidson NE, et al. Locoregional failure ten years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol 1999;17:1689–1700
Senofsky GM, Moffat FL, Davis K, et al. Total axillary lymphadenectomy in the management of breast cancer. Arch Surg 1991;126:1336–1342
Siegel BM, Mayzel KA, Love SM. Level I and II axillary dissection in the treatment of early stage breast cancer. An analysis of 259 consecutive patients. Arch Surg 1990;125:1144–1147
Arriagada R, Rutqvist LE, Mattsson A, et al. Adequate locoregional treatment for early breast cancer may prevent secondary dissemination. J Clin Oncol 1995;13:2869–2878
Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy: Danish Breast Cancer Cooperative Group 82b Trial. N Eng J Med 1997;337:949–955
Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Eng J Med 1997;337:956–962
Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high–risk postmenopausal breast cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group 82c randomized trial. Lancet 1999;353:1641–1648
Whelan TJ, Julian J, Wright J, et al. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol 2000;18:1220–1229
Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia Randomized Trial. J Natl Cancer Inst 2005;97:116–126
Katz A, Strom EA, Buchholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicine-based chemotherapy: Implications for postoperative irradiation. J Clin Oncol 2000;18:2817–2827
Beenken SW, Urist MM, Zhang Y, et al. Axillary lymph node status, but not tumor size, predicts locoregional recurrence and overall survival after mastectomy for breast cancer. Ann Surg 2003;237:732–739
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow up of randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Eng J Med 2002;347:1233–1241
Deckers PJ. Axillary dissection in breast cancer: when, why, how much, and for how long? Another operation soon to be extinct? J Surg Oncol 1991;48:217–219
Henderson IC. Axillary surgery: clinical judgment required. J Clin Oncol 2006;24:325–326
Orr RK. The impact of prophylactic axillary node dissection on breast cancer survival: a Bayesian metaanalysis. Ann Surg Oncol 1999;61:109–116
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–2106
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687–1717
Olivotto IA, Truong PT. Postmastectomy radiation therapy: who needs it? J Clin Oncol 2004;22:4237–4239
Parmigiani G, Berry DA, Winer EP, et al. Is axillary lymph node dissection indicated for early stage breast cancer? A decision analysis. J Clin Oncol 1999;17:1465–1473
Schrenk P, Rieger R, Shamiyeh A, et al. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer 2000;88:608–614
Louis-Sylvestre C, Clough K, Asselain B, et al. Axillary treatment in conservative management of operable breast cancer: Dissection or radiotherapy? Results of a randomized study with 15 years of follow up. J Clin Oncol 2004;22:97–101
Katz A, Strom EA, Buchholz TA, et al. The influence of pathologic tumor characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys 2001;50:735–742
Wallgren A, Bonetti M, Gelber RD, et al. Risk factors for locoregional recurrence among breast cancer patients: results from International Breast Cancer Study Group Trials I Through VII. J Clin Oncol 2003;21:1205–1213
Voogd AC, Nielsen M, Peterse JL, et al., Danish Breast Cancer Cooperative Group, Breast Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol 2001;19:1688–1697
Woodward WA, Strom EA, Tucker SL, et al. Locoregional recurrence after doxorubicine-based chemotherapy and postmastectomy implications for breast cancer patients with early-stage disease and predictors for recurrence after postmastectomy radiation. Int J Radiat Oncol Biol Phys 2003;57:336–344
Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy. Results of five National Surgical Adjuvant Breast and Bowel Project Randomized Clinical Trials. J Clin Oncol 2004;22:4247–4254
Jager JJ, Volovics L, Schouten LJ, et al. Locoregional recurrences after mastectomy in breast cancer: prognostic factors and implications for postoperative irradiation. Radiother Oncol 1999;50:267–275
Fisher BJ, Perera FE, Cooke AL, et al. Long-term follow-up axillary node-positive breast cancer patients receiving adjuvant systemic therapy alone: patterns of recurrence. Int J Radiat Oncol Biol Phys 1997;38:541–550
Cheng JCH, Chen CM, Liu MC, et al. Locoregional failure of postmastectomy patients with 1–3 positive axillary lymph nodes without adjuvant radiotherapy. Int J Radiat Oncol Biol Phys 2002;52:980–988
Gruber G, Bonetti M, Nasi ML, et al. Prognostic value of extracapsular tumor spread for locoregional control in premenopausal patients with node-positive breast cancer treated with classical cyclophosphamide, methotrexate, and fluorouracil: long–term observations from International Breast Cancer Study Group Trial VI. J Clin Oncol 2005;23:7089–7097
Katz A, Buchholz TA, Thames H, et al. Recursive partitioning analysis of locoregional recurrence patterns following mastectomy: implication for adjuvant irradiation. Int J Radiat Oncol Biol Phys 2001;50:397–403
Van der Wal BCH, Butzelaar RMJM, van der Meij S, et al. Axillary lymph node ratio and total number of removed lymph nodes: predictors of survival in stage I and II breast cancer. Eur J Surg Oncol 2002;28:481–489
Voordeckers M, Vinh-Hung V, Van de Sten J, et al. The lymph node ratio as prognostic factor in node positive breast cancer. Radiother Oncol 2004;70:225–230
Haris JR, Halpin-Murphy P, McNeese M, et al. Consensus statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys 1999;44:989–990
Van der Hage JA, Putter H, Bonnema J, et al., EORTC Breast Cancer Group. Impact of locoregional treatment on the early-stage breast cancer patients: a retrospective analysis. Eur J Cancer 2003;39:2192–2199
Schoppmann SF, Bayer G, Aumayr K, et al. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg 2004;240:306–312
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yildirim, E., Berberoglu, U. Lymph Node Ratio is More Valuable than Level III Involvement for Prediction of Outcome in Node-Positive Breast Carcinoma Patients. World J. Surg. 31, 276–289 (2007). https://doi.org/10.1007/s00268-006-0487-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-006-0487-5