Abstract
Background
The importance of the IGF system in HPT has been previously demonstrated. Additionally, the role of vitamin A in HPT has been reported. Retinoic acid (RA), a derivative of vitamin A, is a ligand for the IGF II receptor (IGF2R). We have evaluated the interactions of RA with the IGF system in a primary parathyroid cell culture model.
Materials and Methods
Primary cell cultures were prepared from nine patients. Following adhesion, the cells were transferred to serum-free medium and dosed once with growth factors ± RA for 96 hours. Proliferation was assessed by measuring tritiated thymidine incorporation.
Results
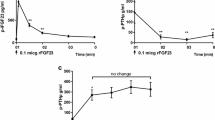
Compared with the control group (100%), both IGF I and II increased DNA synthesis significantly. Retinoic acid significantly reduced the basal DNA synthesis to 82.2% ± 4.2% compared with control (P < 0.05). Retinoic acid ×10−5 M completely abrogated the proliferative actions of IGF II (70.2% ± 9.7%, P < 0.05) but had no significant effect on the IGF I response (P > 0.05). To evaluate the role of IGF2R or IGFBPs in mediating the actions of RA, the IGF II analogs [Leu27]IGF II (10–20-fold reduced IGF I receptor affinity) and des(1–6) IGF II (lower IGFBP binding affinity) were used. The IGF II inhibitory effect of RA was enhanced in the presence of analogs [Leu27]IGF II (P = 0.052) but not with des(1–6)IGF II (P > 0.05), compared with wild-type IGF II.
Conclusions
These data implicate a novel antiproliferative role for RA in enhancing the pericellular clearance of IGF II via the IGF2R preventing ligand activation of the IGF I receptor. This may have broader implications for RA effects in other tumors.







Similar content being viewed by others
References
Hellman P, Liu W, Westin G, et al. Vitamin D and retinoids in parathyroid glands (review). Int J Mol Med 1999;3:355–361
Liu W, Hellman P, Li Q, et al. Biosynthesis and function of all-trans- and 9-cis-retinoic acid in parathyroid cells. Biochem Biophys Res Commun 1996;229:922–929
Li Y, Lin B, Agadir A, et al. Molecular determinants of AHPN (CD437)-induced growth arrest and apoptosis in human lung cancer cell lines. Mol Cell Biol 1998;18:4719–4731
Biasco G, Paganelli GM. European trials on dietary supplementation for cancer prevention. Ann N Y Acad Sci 1999;889:152–156
Lotan R. Retinoids and chemoprevention of aerodigestive tract cancers. Cancer Metastasis Rev 1997;16:349–356
Stein G, Schone S, Geinitz D, et al. No tissue level abnormality of vitamin A concentration despite elevated serum vitamin A of uremic patients. Clin Nephrol 1986;25:87–93
Mira YLR, Zheng WL, Kuppumbatti YS, et al. Retinol conversion to retinoic acid is impaired in breast cancer cell lines relative to normal cells. J Cell Physiol 2000;185:302–309
Murakami K, Matsuura T, Hasumura S, et al. Involvement of insulin-like growth factor binding protein-3 in the retinoic acid receptor-alpha-mediated inhibition of hepatocellular carcinoma cell proliferation. Cancer Lett 2000;151:63–70
Oosterlaken-Dijksterhuis MA, Kwant MM, Slob A, et al. IGF-I and retinoic acid regulate the distribution pattern of IGFBPs synthesized by the canine mammary tumor cell line CMT-U335. Breast Cancer Res Treat 1999;54:11–23
Kang JX, Bell J, Leaf A, et al. Retinoic acid alters the intracellular trafficking of the mannose-6-phosphate/insulin-like growth factor II receptor and lysosomal enzymes. Proc Natl Acad Sci USA 1998;95:13687–13691
Byrd JC, Devi GR, de Souza AT, et al. Disruption of ligand binding to the insulin-like growth factor II/mannose 6-phosphate receptor by cancer-associated missense mutations. J Biol Chem 1999;274:24408–24416
York SJ, Arneson LS, Gregory WT, et al. The rate of internalization of the mannose 6-phosphate/insulin-like growth factor II receptor is enhanced by multivalent ligand binding. J Biol Chem 1999;274:1164–1171
Melhus H, Li Q, Nordlinder H, et al. Expression of cellular retinol- and retinoic acid-binding proteins in normal and pathologic human parathyroid glands. Endocr Pathol 2001;12:423–427
Liu XW, Gong LJ, Guo LY, et al. The Wilms’ tumor gene product WT1 mediates the down-regulation of the rat epidermal growth factor receptor by nerve growth factor in PC12 cells. J Biol Chem 2001;276:5068–5073
Baserga R, Resnicoff M, D’Ambrosio C, et al. The role of the IGF-I receptor in apoptosis. Vitam Horm 1997;53:65–98
LeRoith D, Baserga R, Helman L, et al. Insulin-like growth factors and cancer. Ann Intern Med 1995;122:54–59
Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev 1996;76:1005–1026
Wong C, Lai T, Hilly JM, et al. Selective estrogen receptor modulators inhibit the effects of insulin-like growth factors in hyperparathyroidism. Surgery 2002;132:998–1006; discussion 1006–1007
Jones HE, Eaton CL, Barrow D, et al. Response of cell growth and retinoic acid receptor expression to retinoic acid in ceoplastic and non-neoplastic prostate cell lines. Prostate. 1997;30:174–182
De Leon DD, Terry C, Asmerom Y, et al. Insulin-like growth factor II modulates the routing of cathepsin D in MCF-7 breast cancer cells. Endocrinology 1996;137:1851–1859
De Leon DD, Issa N, Nainani S, et al. Reversal of cathepsin D routing modulation in MCF-7 breast cancer cells expressing antisense insulin-like growth factor II (IGF-II). Horm Metab Res 1999;31:142–147
Brown SB, Brierley TT, Palanisamy N, et al. Vitamin D receptor as a candidate tumor-suppressor gene in severe hyperparathyroidism of uremia. J Clin Endocrinol Metab 2000;85:868–872
Thakker RV, Fraher LJ, Adami S, et al. Circulating concentrations of 1,25-dihydroxyvitamin D3 in patients with primary hyperparathyroidism. Bone Miner 1986;1:137–144
MacDonald PN, Ritter C, Brown AJ, et al. Retinoic acid suppresses parathyroid hormone (PTH) secretion and PreproPTH mRNA levels in bovine parathyroid cell culture. J Clin Invest 1994;93:725–730
Stewart LV, Thomas ML. Retinoids differentially regulate the proliferation of colon cancer cell lines. Exp Cell Res. 1997;233:321–329
Martinet N, Alla F, Farre G, et al. Retinoic acid receptor and retinoid X receptor alterations in lung cancer precursor lesions. Cancer Res 2000;60:2869–2875
Sun SY, Wan H, Yue P, et al. Evidence that retinoic acid receptor beta induction by retinoids is important for tumor cell growth inhibition. J Biol Chem 2000;275:17149–17153
Leid M, Kastner P, Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci 1992;17:427–433
Brand C, Segard P, Plouvier P, et al. Selective alteration of gene expression in response to natural and synthetic retinoids. BMC Pharmacol 2002;2:13
Braulke T. Type-2 IGF receptor: a multi-ligand binding protein. Horm Metab Res 1999;31:242–246
Altenahr E, Dietel M, Dorn G, et al. The effect of 1,25-dihydroxycholecalciferol on the parathyroid hormone secretion of porcine parathyroid glands and human parathyroid adenomas in vitro. Acta Endocrinol (Copenh) 1977;86:533–538
Acknowledgments
This research was supported by grants from The Royal College of Surgeons Research Fellowship, Mason’s Medical Research Foundation, the Hamamelis Trust, Royal Society, the Lord Dowding Fund for Humane Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, C.K., Lai, T., Holly, J.M. et al. The Effects of Retinoic Acid on the Insulin-like Growth Factor Axis in Primary Tissue Culture from Hyperparathyroidism. World J. Surg. 30, 714–720 (2006). https://doi.org/10.1007/s00268-005-0340-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-005-0340-2