Abstract
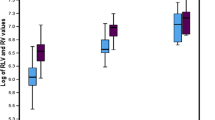
Liver regeneration after donor hepactectomy offers a unique insight into the process of liver regeneration in normal livers. As the liver restores itself, concurrent splenic enlargement occurs. There are many theories about why this phenomenon takes place: some investigators have proposed a relative portal hypertension that leads to splenic congestion or, perhaps, the presence of a common growth factor that induces both the liver and spleen to enlarge. Between the months of June 2001 and May 2004, 112 live donor liver transplants (LDLTs) were performed in Chang Gung Memorial Hospital, Kaohsiung, Taiwan. The total number of donor hepatectomies performed during this period was 113, however, because one of the cases required dual donors. Of our 113 donors, we eventually analyzed the data of 109; 4 patients were lost to follow-up 6 months later and were excluded from our study. The average age of our donor population was 32.32 ± 8.48 years. The mean liver volume at donation was noted to be 1207.72 ± 219.95 cm3, and 6 months later, it was 1027.18 ± 202.41 cm3. Expressed as a percentage of the original volume, the mean liver volume 6 months after hepatectomy was 90.70% ± 12.47% in this series. For right graft donors, mean liver volume after 6 months was 89.68% ± 12.37% of the original liver volume, whereas that for left graft donors was 91.99% ± 12.6%. Only 26 of the 109 (23.85%) donors were able to achieve full regeneration 6 months post-donation. Notably, liver function profiles of all donors were normal when measured 6 months after operation. The average splenic volume at donation as measured by computed tomography (CT) volumetry was 159 ± 58 cm3, and the splenic volume 6 months post-donation was 213 ± 85 cm3. There was a mean increment in splenic volume of 35% ± 28% 6 months after donation. The blood profiles of the donors were monitored; particular attention was given to platelet levels and liver function tests, and these were found to be within normal limits 6 months after operation. Of note, splenic enlargement was significantly greater among right-sided donors than their left-sided counterparts. Greater splenic enlargement was also observed in those donors who achieved full liver regeneration at their evaluation 6 months postoperatively than in those who did not. Although original liver volume was not re-established in most patients 6 months after liver donation, there seemed to have been no untoward effects to the donor. The factors that affect liver regeneration are complex and myriad. Although there is splenic enlargement at 6 months post-donation in donors of LDLT, there are no untoward effects of this enlargement.
Similar content being viewed by others
References
Nakagami M, Morimoto T, Itoh K, et al. Patterns of restoration of remnant liver volume after graft harvesting in donors for living related liver transplantation. Transplant. Proc. 1998;30:195–199
Nagasue N, Yukaya N, Ogawa Y, et al. Human liver regeneration after major hepatic resection. Ann Surg 1987;206:30
Tanaka W, Yamanaka N, Oriyama T, et al. Multivariate analysis of liver regenerative capacity after hepactectomy in humans. J. Hepatobil. Pancreat. Surg. 1997;4:78–82
Yamanaka N, Okamoto E, Kawamura E, et al. Dynamics of normal and injured liver human liver regeneration after hepactectomy as assessed on the basis of computed tomography and liver function. Hepatology 1993;18:79–85
Bucher NH. Experimental aspects of hepatic regeneration. N. Engl. J. Med. 1967; 277:686
Fausto N. Liver regeneration: from laboratory to clinic. Liver Transplant. 2001;7:835
Court FG, Wemyss-Holden SA, Dennison AR, et al. The mystery of liver regeneration. Br. J. Surg. 2002;89:1089
Olthoff KM. Molecular pathways of regeneration and repair after liver transplantation. World J. Surg. 2002;26:831
Marcos A, Fischer RA, Ham JM, et al. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation 2000;69:1375–1379
Pomfret EA, Pomposelli JJ, Gordon FD, et al. Liver regeneration and surgical outcome in donors of right lobe liver grafts. Transplantation 2003;76:5–10
Kawasaki S, Makuuchi M, Matsunami H, et al. Living related liver transplantation in adults. Ann Surg 1998;227–269
Zollinger RM, Zollinger RM Jr. Left hepatic lobectomy. In Zollinger RM, Zollinger RM Jr, (editors), Atlas of Surgical Operations, 4th edition, New York, Macmillian, 1990; 172
Akimaru K, Onda M, Tajiri T, et al. Hypersplenism induced by hepatectomy. Hepato-Gastroenterology 2001;48:1170–1175
Ando H, Nagino M, Arai T, et al. Changes in splenic volume during liver regeneration. World J. Surg. 2004;28:977–981
Tanaka W, Yamanaka N, Oriyama T. Multivariate analysis of liver regenerative capacity after hepactectomy in humans. J. Hepatobiliary Pancreatic Surg. 1997;4:78–82
Miyagawa S, Kawasaki S, Noike T, et al. Liver regeneration after extended right hemihepactectomy in patients with hilar or diffuse bile duct carcinoma. Hepatogastroenterology 1999;46:364–368
Nagino M, Ando M, Kamiya J, et al. Liver regeneration after major hepactectomy for biliary cancer. Br. J. Surg. 2001;88:1084–1091
Jacob HS, Macdonald RA, Jandl JH. Regulation of spleen growth and sequestering function. J. Clin. Invest. 1963;42:1476–1490
Tavassoli M. Limitation of splenic growth as studied by heterotopic splenic implants. Blood 1975;46:631–635
Chen YS, Cheng YF, De Villa VH, et al. Evaluation of living liver donors. Transplantation 2003;75(Suppl 3):S16–S19
Chen CL, Chen YS, De Villa VH, et al. Minimal blood loss living donor hepatectomy. Transplantation 2000;69:2580–2586
Greene AK, Wiener S, Puder M, et al. Endothelial directed hepatic regeneration after partial hepactectomy. Ann. Surg. 2002;236:530–534
Francavilla A, Polimeno L, Dileo A, et al. The effect of estrogen and tamoxifen on hepatocyte proliferation in vivo and in vitro. Hepatology 1989;9–614
Chiu J, Lin L, Chi W, et al. Estrogen therapy for hepactectomy with poor liver function? Med Hypotheses 2002;58:516
Nagasue N, Yukaya H, Ogawa Y, et al. Portal pressure following partial to extensive hepatic resection in patients with and without cirrhosis of the liver. Ann. Chir. Gynecol. 1983;72:18–22
Ueda S, Yamanoi A, Hishikawa Y, et al. Transforming growth factor beta-1 released from the spleen exerts a growth inhibitory effect on liver regeneration in rats. Lab. Invest. 2003;83:1595–1603
Kaido T, Oe H, Yoshikawa A, et al. Expressions of molecules associated with hepatocyte growth factor activation after hepatectomy in liver cirrhosis. Hepato-Gastroenterology 2004;51:547–551
Charters AC, Oakes DD, Froechlich JP. Effect of hepatectomy on mitotic activity in rat spleen. J. Surg. Res. 1980;29:331–337
Rosenkranz E, Charters AC, Orloff MJ. Regeneration in rat liver injured by carbon tetrachloride. Surg. Forum 1975;26:411–412
Tomiya T, Tani M, Yamada S, et al. Serum hepatocyte growth factor levels in hepactomised and nonhepactomised surgical patients. Gastroenterology 1992;103:1621–1624
Nishizaki T, Takenaka K, Yoshizumi T, et al. Alteration in levels of human hepatocyte growth factor following hepactectomy. J. Am. Coll. Surg. 1995;181:6–10
Sato K, Tanaka M, Tanikawa K. The effect of spleen volume on liver regeneration after hepatectomy—a clinical study of liver and spleen volumes by computed tomography. Hepatogastroenterology. 1995;42:961–965
Acknowledgements
This work is supported in part by program project grant NHRI-EX 93-228SP from the National Health Research Institutes, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibrahim, S., Chen, CL., Wang, CC. et al. Liver Regeneration and Splenic Enlargement in Donors after Living-Donor Liver Transplantation. World J. Surg. 29, 1658–1666 (2005). https://doi.org/10.1007/s00268-005-0101-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-005-0101-2