Abstract
Background
In plastic surgery, autologous fat grafts (AFG) play an important role because of their abundant supply, biocompatibility, and low rejection rate. However, the lower retention rate of fat grafts limits their widespread use. Brown adipose tissue (BAT) can promote angiogenesis and regulate the level of associated inflammation. This study explored whether BAT has a facilitative effect on fat graft retention.
Methods
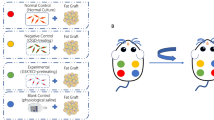
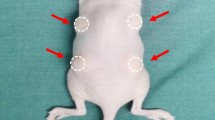
We obtained white adipose tissue (WAT) from c57 mice and combined it with either BAT from c57 mice or phosphate-buffered saline (PBS) as a control. These mixtures were injected subcutaneously into the back of thymus-free nude mice. After 12 weeks, fat grafts were harvested, weighed, and analyzed.
Results
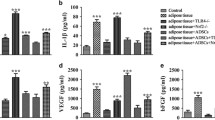
We found that the BAT-grafted group had higher mass retention, more mature adipocytes, and higher vascularity than the other group. Further analysis revealed that BAT inhibited M1 macrophages; down-regulated IL-6, IL-1β, and TNF-β; upregulated M2 macrophages and Vascular endothelial growth factor-A (VEGFA); and promoted adipocyte regeneration by inhibiting the Wnt/β-catenin pathway, which together promoted adipose graft retention.
Conclusion
The study demonstrated that BAT improved adipose graft retention by promoting angiogenesis, inhibiting tissue inflammation levels and the Wnt/β-catenin pathway.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.




Similar content being viewed by others
References
Sun JM, Ho CK, Gao Y, Chong CH, Zheng DN, Zhang YF, Yu L (2021) Salvianolic acid-B improves fat graft survival by promoting proliferation and adipogenesis. Stem Cell Res Ther 12:1–16
Bayram Y, Sezgic M, Karakol P, Bozkurt M, Filinte GT (2019) The use of autologous fat grafts in breast surgery: a literature review. Arch Plast Surg 46(06):498–510
Coleman SR (2020) Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg 44(4):1268–1272
Yuan X, Hu T, Zhao H, Huang Y, Ye R, Lin J, Chen ZJ (2016) Brown adipose tissue transplantation ameliorates polycystic ovary syndrome. Proceed Nat Acad Sci 113(10):2708–2713
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1):277–359
Okamatsu-Ogura Y, Kuroda M, Tsutsumi R, Tsubota A, Saito M, Kimura K, Sakaue H (2020) UCP1-dependent and UCP1-independent metabolic changes induced by acute cold exposure in brown adipose tissue of mice. Metabolism 113:154396
Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, Cao Y (2009) Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metabol 9(1):99–109
Neels JG, Thinnes T, Loskutoff DJ (2004) Angiogenesis in an in vivo model of adipose tissue development. FASEB J 18(9):983–985
Yi CG, Xia W, Zhang LX, Zhen Y, Shu MG, Han Y, Guo SZ (2007) VEGF gene therapy for the survival of transplanted fat tissue in nude mice. J Plast Reconstr Aesthet Surg 60(3):272–278
Elias I, Franckhauser S, Ferré T, Vilà L, Tafuro S, Muñoz S, Bosch F (2012) Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 61(7):1801–1813
Cai J, Feng J, Liu K, Zhou S, Lu F (2018) Early macrophage infiltration improves fat graft survival by inducing angiogenesis and hematopoietic stem cell recruitment. Plast Reconstr Surg 141(2):376–386
Yu Q, Cai Y, Huang H et al (2018) Co-transplantation of nanofat enhances neovascularization and fat graft survival in nude mice. Aesthetic Surg J 38(6):667–675
Song Y et al (2018) Usnic acid inhibits hypertrophic scarring in a rabbit ear model by suppressing scar tissue angiogenesis. Biomed Pharmacother 108:524–530
Garin-Shkolnik T, Rudich A, Hotamisligil GS, Rubinstein M (2014) FABP4 attenuates PPARγ and adipogenesis and is inversely correlated with PPARγ in adipose tissues. Diabetes 63(3):900–911
Reggio A, Rosina M, Palma A, Cerquone Perpetuini A, Petrilli LL, Gargioli C, Cesareni G (2020) Adipogenesis of skeletal muscle fibro/adipogenic progenitors is affected by the WNT5a/GSK3/β-catenin axis. Cell Death Differ 27(10):2921–2941
Zuo Q, He J, Zhang S, Wang H, Jin G, Jin H, Qin W (2021) PPARγ Coactivator-1α suppresses metastasis of hepatocellular carcinoma by inhibiting Warburg effect by PPARγ–dependent WNT/β-catenin/pyruvate dehydrogenase kinase isozyme 1 axis. Hepatology 73(2):644–660
Nishimura T, Hashimoto H, Nakanishi I, Furukawa M (2000) Microvascular angiogenesis and apoptosis in the survival of free fat grafts. Laryngoscope 110(8):1333–1338
Villarroya J, Cereijo R, Gavaldà-Navarro A, Peyrou M, Giralt M, Villarroya F (2019) New insights into the secretory functions of brown adipose tissue. J Endocrinol 243(2):R19–R27
Villarroya F, Cereijo R, Villarroya J, Giralt M (2017) Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 13(1):26–35
Wang GX, Zhao XY, Lin JD (2015) The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol Metab 26(5):231–237
Tran TT, Kahn CR (2010) Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol 6(4):195–213
Liu X, Wang S, You Y, Meng M, Zheng Z, Dong M, Jin W (2015) Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology 156(7):2461–2469
Payab M, Abedi M, Foroughi Heravani N, Hadavandkhani M, Arabi M, Tayanloo-Beik A, Arjmand B (2021) Brown adipose tissue transplantation as a novel alternative to obesity treatment: a systematic review. Int J Obes 45(1):109–121
Wang W, Seale P (2016) Control of brown and beige fat development. Nat Rev Mol Cell Biol 17(11):691–702
Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Shima DT (1999) Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med 5(5):495–502
Khouri Jr RK, Khouri RE, Lujan-Hernandez JR, Khouri KR, Lancerotto L, Orgill DP (2014) Diffusion and perfusion: the keys to fat grafting. Plast Reconstr Surg Glob Open 2(9)
Wu M, Li Y, Wang Z, Feng J, Wang J, Xiao X, Dong Z (2020) Botulinum toxin a improves supramuscular fat graft retention by enhancing angiogenesis and adipogenesis. Dermatol Surg 46(5):646–652
Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K, Goodman SB (2019) Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 196:80–89
Lee P, Swarbrick MM, Ho KK (2013) Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev 34(3):413–438
Ferrante AW Jr (2013) The immune cells in adipose tissue. Diabetes Obes Metab 15(s3):34–38
Kepple JD, Barra JM, Young ME, Hunter CS, Tse HM (2022) Islet transplantation into brown adipose tissue can delay immune rejection. JCI Insight 7(4)
Espinoza-Jiménez A, Peon AN, Terrazas LI (2012) Alternatively activated macrophages in types 1 and 2 diabetes. Med Inflamm 2012:815953
Zhang S, Liu Y, Zhang X, Zhu D, Qi X, Cao X, Li Z (2018) Prostaglandin E2 hydrogel improves cutaneous wound healing via M2 macrophages polarization. Theranostics 8(19):5348
Li W, Zhang X, Wu F, Zhou Y, Bao Z, Li H, Zhao S (2019) Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Disease 10(12):918
Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM (2002) C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev 16(1):22–26
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810
Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibañez G, MacDougald OA (2012) Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 50(2):477–489
Funding
This study is suppoted by Natural Science Foundation of Anhui Province (2008085QC112).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Informed Consent
For this type of study, informed consent was not required.
Human and Animal Rights
We followed all guidelines for the care and use of animals. There was no research on human participants in this investigation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, Y., Li, H., Bao, Q. et al. Brown Adipose Tissue Promotes Autologous Fat Grafts Retention Possibly Through Inhibiting Wnt/β-Catenin Pathway. Aesth Plast Surg 48, 1817–1824 (2024). https://doi.org/10.1007/s00266-024-03888-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-024-03888-4