Abstract
Background
Nanofat and lipoconcentrate contain adipose-derived stem cells and growth factors, and have wide clinical applications in the regenerative field. This study aimed to investigate the microenvironmental changes associated with nanofat and lipoconcentrate.
Methods
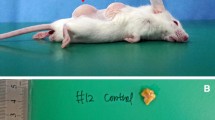
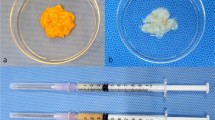
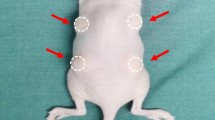
Conventional fat, nanofat, or lipoconcentrate (0.2 mL each, n = 5 per group) were injected subcutaneously into the dorsal flanks of athymic nude mice. The graft weights were measured at postoperative week 4; the grafts and their overlying skin were used for histological analyses.
Results
Weights of the lipoconcentrate grafts were significantly greater than those of the conventional fat (p < 0.05) and nanofat (p < 0.01) grafts. There was no significant difference in inflammation, oil cysts, and fibrosis between the conventional fat and nanofat groups. Histological examination of the lipoconcentrate grafts showed less macrophage infiltration and the formation of fibrosis and oil cysts. Additionally, adipogenesis and angiogenesis were induced more in the lipoconcentrate grafts than in the nanofat grafts (p < 0.01). Lipoconcentrate and nanofat improved dermal thickness (p < 0.001 and p < 0.01, respectively, versus the baseline).
Conclusion
Lipoconcentrate grafts had greater volume and shape retention than conventional fat and nanofat grafts. They had better histological structure and acted as scaffolds for adipogenesis and angiogenesis. Both products showed regenerative effects on dermal thickness; however, only lipoconcentrate grafts had the required volume and regenerative effects, allowing it to serve as a novel adipose-free grafting method for facial rejuvenation and contouring.
No Level Assigned
This journal requires that authors assign a level of evidence to each submission to which Evidence-Based Medicine rankings are applicable. This excludes Review Articles, Book Reviews, and manuscripts that concern Basic Science, Animal Studies, Cadaver Studies, and Experimental Studies. For a full description of these Evidence-Based Medicine ratings, please refer to Table of Contents or the online Instructions to Authors www.springer.com/00266.







Similar content being viewed by others
References
Coleman SR (2006) Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. https://doi.org/10.1097/01.prs.0000234610.81672.e7
Gentile P, Piccinno M, Calabrese C (2019) Characteristics and potentiality of human adipose-derived stem cells (hASCs) obtained from enzymatic digestion of fat graft. Cells 8:282. https://doi.org/10.3390/cells8030282
Jeyaraman M, Muthu S, Sharma S et al (2021) Nanofat: a therapeutic paradigm in regenerative medicine. World J Stem Cells 13:1733–1746. https://doi.org/10.4252/wjsc.v13.i11.1733
Atiyeh B, Ghieh F, Oneisi A (2021) Nanofat cell-mediated anti-aging therapy: evidence-based analysis of efficacy and an update of stem cell facelift. Aesthetic Plast Surg 45:2939–2947. https://doi.org/10.1007/s00266-021-02353-w
Trivisonno A, Alexander RW, Baldari S et al (2019) Intraoperative strategies for minimal manipulation of autologous adipose tissue for cell- and tissue-based therapies: concise review. Stem Cells Transl Med 8:1265–1271
Tonnard P, Verpaele A, Peeters G et al (2013) Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 132:1017–1026
Kim W-S, Park B-S, Park S-H et al (2009) Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci 53:96–102. https://doi.org/10.1016/j.jdermsci.2008.08.007
LeBert DC, Squirrell JM, Rindy J et al (2015) Matrix metalloproteinase 9 modulates collagen matrices and wound repair. Development 142:2136–2146. https://doi.org/10.1242/dev.121160
Kim B-S, Rongisch R, Hager S et al (2015) Macrophage migration inhibitory factor in acute adipose tissue inflammation. PLoS ONE 10:e0137366. https://doi.org/10.1371/journal.pone.0137366
Sanchez-Macedo N, McLuckie M, Grunherz L, Lindenblatt N (2022) Protein profiling of mechanically processed lipoaspirates: discovering wound healing and antifibrotic biomarkers in nanofat. Plast Reconstr Surg 150:341E-354E. https://doi.org/10.1097/PRS.0000000000009345
Dolivo DM, Larson SA, Dominko T (2017) Fibroblast growth factor 2 as an antifibrotic: antagonism of myofibroblast differentiation and suppression of pro-fibrotic gene expression. Cytokine Growth Factor Rev 38:49–58. https://doi.org/10.1016/j.cytogfr.2017.09.003
Pallua N, Grasys J, Kim BS (2018) Enhancement of progenitor cells by two-step centrifugation of emulsified lipoaspirates. Plast Reconstr Surg 142:99–109. https://doi.org/10.1097/PRS.0000000000004495
Tran VVT, Jin X, Hong KY, Chang H (2023) Effects of nanofat in plastic and reconstructive surgery: a systematic review. Plast Reconstr Surg. https://doi.org/10.1097/PRS.0000000000010905
Liu T, Fu S, Wang Q et al (2021) Browning of white adipocytes in fat grafts associated with higher level of necrosis and type 2 macrophage recruitment. Aesthet Surg J 41:NP1092–NP1101. https://doi.org/10.1093/asj/sjab144
Li F, Guo W, Li K et al (2015) Improved fat graft survival by different volume fractions of platelet-rich plasma and adipose-derived stem cells. Aesthet Surg J 35:319–333. https://doi.org/10.1093/asj/sju046
Yu Q, Cai YZ, Huang H et al (2018) Co-transplantation of nanofat enhances neovascularization and fat graft survival in nude mice. Aesthet Surg J 38:667–675. https://doi.org/10.1093/asj/sjx211
Hong KY, Yim S, Kim HJ et al (2018) The fate of the adipose-derived stromal cells during angiogenesis and adipogenesis after cell-assisted lipotransfer. Plast Reconstr Surg 141:365–375. https://doi.org/10.1097/PRS.0000000000004021
Park BY, Wu D, Kwon KR et al (2023) Implantation and tracing of green fluorescent protein-expressing adipose-derived stem cells in peri-implant capsular fibrosis. Stem Cell Res Ther 14:22. https://doi.org/10.1186/s13287-023-03248-0
Eto H, Kato H, Suga H et al (2012) The fate of adipocytes after nonvascularized fat grafting. Plast Reconstr Surg 129:1081–1092. https://doi.org/10.1097/PRS.0b013e31824a2b19
Vaittinen M, Männistö V, Käkelä P et al (2017) Interorgan cross talk between fatty acid metabolism, tissue inflammation, and FADS2 genotype in humans with obesity. Obesity 25:545–552. https://doi.org/10.1002/oby.21753
Namgaladze D, Brüne B (2016) Macrophage fatty acid oxidation and its roles in macrophage polarization and fatty acid-induced inflammation. Biochim Biophys Acta 1861:1796–1807. https://doi.org/10.1016/j.bbalip.2016.09.002
Lindhorst A, Raulien N, Wieghofer P et al (2021) Adipocyte death triggers a pro-inflammatory response and induces metabolic activation of resident macrophages. Cell Death Dis 12:579. https://doi.org/10.1038/s41419-021-03872-9
Triantafyllou E-A, Mylonis I, Simos G, Paraskeva E (2019) Hypoxia induces pro-fibrotic and fibrosis marker genes in hepatocellular carcinoma cells independently of inflammatory stimulation and the NF-κΒ pathway. Hypoxia 7:87–91. https://doi.org/10.2147/HP.S235967
Sano H, Orbay H, Terashi H et al (2014) Acellular adipose matrix as a natural scaffold for tissue engineering. J Plast Reconstr Aesthet Surg 67:99–106. https://doi.org/10.1016/j.bjps.2013.08.006
Wu I, Nahas Z, Kimmerling KA et al (2012) An injectable adipose matrix for soft-tissue reconstruction. Plast Reconstr Surg 129:1247–1257. https://doi.org/10.1097/PRS.0b013e31824ec3dc
Kato H, Mineda K, Eto H et al (2014) Degeneration, regeneration, and cicatrization after fat grafting. Plast Reconstr Surg 133:303e–313e. https://doi.org/10.1097/PRS.0000000000000066
Zheng HJ, Yu ZY, Deng MW et al (2019) Fat extract improves fat graft survival via proangiogenic, anti-apoptotic and pro-proliferative activities. Stem Cell Res Ther 10:174. https://doi.org/10.1186/s13287-019-1290-1
Hong KY (2020) Fat grafts enriched with adipose-derived stem cells. Arch Craniofac Surg 21:211–218. https://doi.org/10.7181/acfs.2020.00325
Righesso R, Piccinini P, Uebel C (2021) A combined approach for fast nanofat microneedling. J Cutan Aesthet Surg 14:248–255. https://doi.org/10.4103/JCAS.JCAS_142_20
Uyulmaz S, Macedo NS, Rezaeian F et al (2018) Nanofat grafting for scar treatment and skin quality improvement. Aesthet Surg J 38:421–428. https://doi.org/10.1093/asj/sjx183
Kim J-H, Jung M, Kim H-S et al (2011) Adipose-derived stem cells as a new therapeutic modality for ageing skin. Exp Dermatol 20:383–387. https://doi.org/10.1111/j.1600-0625.2010.01221.x
Xu P, Yu Q, Huang HZ et al (2018) Nanofat increases dermis thickness and neovascularization in photoaged nude mouse skin. Aesthetic Plast Surg 42:343–351. https://doi.org/10.1007/s00266-018-1091-4
Suszynski TM, Sieber DA, Van Beek AL, Cunningham BL (2015) Characterization of adipose tissue for autologous fat grafting. Aesthet Surg J 35:194–203. https://doi.org/10.1093/asj/sju059
Donofrio L (2008) Techniques in facial fat grafting. Aesthet Surg J 28:681–687. https://doi.org/10.1016/j.asj.2008.09.003
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF- 2022R1C1C1010912 to Ki Yong Hong), and by grant No. 0320230320 from the SNUH Research Fund.
Funding
The authors have no financial or institutional interest in any of the drugs, materials, or devices described in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No benefits have or will be received with the publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
266_2023_3583_MOESM1_ESM.tiff
Images of whole graft sections in the conventional fat, nanofat, and lipoconcentrate groups at postoperative week 4. The conventional fat (left) and nanofat (center) grafts showed large necrotic areas (red dotted line [*]) and numerous large oil cysts (black dotted line [#]). In contrast, lipoconcentrate grafts had fewer oil cysts, inflammation filtration, and fibrosis among the three groups; scale bar, 2 mm. (TIFF 276 kb)
266_2023_3583_MOESM2_ESM.tiff
Immunofluorescence staining of perilipin (green) for pre-adipocytes (yellow arrows), which were small adipocytes with multiple intracellular lipid droplets, in the nanofat, and lipoconcentrate groups at postoperative week 4; scale bar, 100 μm. (TIFF 71 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tran, V.V.T., Hong, K.Y., Jin, X. et al. Histological Comparison of Nanofat and Lipoconcentrate: Enhanced Effects of Lipoconcentrate on Adipogenesis and Angiogenesis. Aesth Plast Surg 48, 752–763 (2024). https://doi.org/10.1007/s00266-023-03583-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-023-03583-w