Abstract
Introduction
The emergence of hyaluronic acid dermal fillers with lidocaine has transformed the minimally invasive treatment of wrinkles, lines and folds of the face. Patients can be treated quickly, painlessly and without the need for large doses of lidocaine. Therefore, it is important to scientifically evaluate the merits of lidocaine-containing products over those without.
Methods
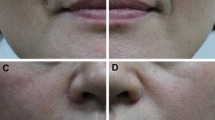
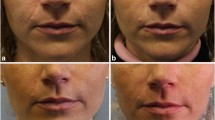
The two products, with (UJU) and without lidocaine (UJ), were randomly injected into nasolabial folds of 75 healthy volunteers with varying skin types in a split face study, age ranging 26–60 years. Only 73 subjects completed the follow-up. There were 68 females and 5 males with medium-to-deep nasolabial folds. All subjects were randomly injected with the two products on one or the other side of the face. Patients were followed up for 9 months.
Results
Both products achieved significant improvement in the wrinkle severity score. Overall results were slightly better with UJU due to ease of injection, lack of pain and avoidance of topical or parenteral anaesthetic. In all other respects, differences in clinical data were not statistically significant. UJU® was preferred by patients and injectors due to less pain during and after injection as compared to UJ® (P < 0.0001). The overall rate of early and late complications with the two products was similar. Duration of maintenance of aesthetic effect between products also showed similarity. Optimum aesthetic effect was maintained in most cases for over 9 months with both products but patients in the 30–50-year age group did better. The patient acceptability rate was much higher with UJU.
Conclusion
Clinical data from this study suggest that performance and outcomes of treatment of medium-to-deep nasolabial folds with UJ and UJU are quite similar. However, treatment with UJU offers enhanced patient comfort and is preferred by patients and injectors.
Level of Evidence I
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.


Similar content being viewed by others
References
Royo de la Torre J, Moreno-Moraga J, Isarría MJ, Muñoz E, Cruz I, Pérez G, Cornejo P (2013) The evaluation of hyaluronic acid, with and without lidocaine, in the filling of nasolabial folds as measured by ultrastructural changes and pain management. J Drugs Dermatol 12(3):e46–52
Prager W, Steinkraus V (2010) A prospective, rater-blind, randomized comparison of the effectiveness and tolerability of Belotero ® Basic versus Restylane ® for correction of nasolabial folds. Eur J Dermatol 20(6):748–752
da Costa A, Biccigo DGZ, de Souza Weimann ET, Mercadante LM, Oliveira PRG, Prebianchi SB, Abdalla BMZ (2017) Durability of three different types of hyaluronic acid fillers in skin: are there differences among biphasic, monophasic monodensified, and monophasic polydensified products? Aesthet Surg J 37(5):573–581. https://doi.org/10.1093/asj/sjw161
World Health Organization (2001) World medical association declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ 79(4):373–374
Vijayananthan A, Nawawi O (2008) The importance of Good Clinical Practice guidelines and its role in clinical trials. Biomed Imaging Interv J 4(1):e5
Day DJ, Littler CM, Swift RW, Gottlieb S (2004) The wrinkle severity rating scale: a validation study. Am J Clin Dermatol 5:49–52
Carruthers A, Carruthers J (2010) A validated facial grading scale: the future of facial ageing measurement tools? J Cosmet Laser Ther 12:235–241
Sharma P, Sharma S (2011) Comparative study of a new dermal filler Uma Jeunesse® and Juvéderm®. J Cosmet Dermatol 10(2):118–125
Levy PM, De Boulle K, Raspaldo H (2009) A split-face comparison of a new hyaluronic acid facial filler containing pre-incorporated lidocaine versus a standard hyaluronic acid facial filler in the treatment of naso-labial folds. J Cosmet Laser Ther 11:169–173
Weinkle SH, Bank DE, Boyd CM et al (2009) A multi-center, double-blind, randomized controlled study of the safety and effectiveness of Juve´derm injectable gel with and without lidocaine. J Cosmet Dermatol 8(3):205–210
Alam M, Dover JS (2007) Management of complications and sequelae with temporary injectable fillers. Plast Reconstr Surg 120(6 Suppl):98S–105S
Pinsky MA, Thomas JA, Murphy DK, Walker PS (2008) Juvederm injectable gel: a multicenter, double-blind, randomized study of safety and effectiveness. Aesthet Surg J 28(5):596–597
Vedamurthy M (2008) IADVL Dermatosurgery task Force. Standard guidelines for the use of dermal fillers. Indian J Dermatol Venereol Leprol 74:S23–S27
Duranti F, Salti G, Bovani B, Calandra M, Rosati ML (1998) Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatol Surg 24:1317–1325
Funt David, Pavicic Tatjana (2013) Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol 6:295–316
Christensen LH (2009) Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Dermatol Surg 35(Suppl 2):1612–1619
Monheit GD, Rohrich RJ (2009) The nature of long-term fillers and the risk of complications. Dermatol Surg 35(Suppl 2):1598–1604
Arsiwala SZ (2010) Safety and persistence of non-animal stabilized hyaluronic acid fillers for nasolabial folds correction in 30 indian patients. J Cutan Aesthet Surg 3(3):156–161
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Sharma, P.P., Sharma, D.K. & Carr, A. Comparative Study of UMA Jeunesse Classic® and UMA Jeunesse Ultra®. Aesth Plast Surg 42, 1111–1118 (2018). https://doi.org/10.1007/s00266-018-1144-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-018-1144-8